Abstract
The BOADICEA breast cancer (BC) risk assessment model and its associated Web Application v3 (BWA) tool are being extended to incorporate additional genetic and non-genetic BC risk factors. From an online survey through the BOADICEA website and UK, Dutch, French and Swedish national genetic societies, we explored the relationships between the usage frequencies of the BWA and six other common BC risk assessment tools and respondents’ perceived importance of BC risk factors. Respondents (N = 443) varied in age, country and clinical seniority but comprised mainly genetics health professionals (82%) and BWA users (93%). Oncology professionals perceived reproductive, hormonal (exogenous) and lifestyle BC risk factors as more important in BC risk assessment compared to genetics professionals (p values < 0.05 to 0.0001). BWA was used more frequently by respondents who gave high weight to breast tumour pathology and low weight to personal BC history as BC risk factors. BWA use was positively related to the weight given to hormonal BC risk factors. The importance attributed to lifestyle and BMI BC risk factors was not associated with the use of BWA or any of the other tools. Next version of the BWA encompassing additional BC risk factors will facilitate more comprehensive BC risk assessment in genetics and oncology practice.
Similar content being viewed by others
Introduction
Breast cancer (BC) is a major public health problem for women with almost 1.7 million new BC diagnoses and 521, 900 BC deaths estimated worldwide in 2012 (DeSantis et al. 2015).
A number of BC risk factors have been identified, including family history, breast density on mammogram, hormonal exposure, reproductive history and lifestyle. A family history of BC suggests the presence of an inherited genetic variant such as those in the BRCA1 and BRCA2 genes which confer a “high” BC susceptibility (Couch et al. 2014). Recently, additional BC genetic risk factors have been identified, including rare variants in genes such as PALB2, CHEK2, ATM (Lee et al. 2016) associated with “moderate” to “high” risk and common “low-risk” variants (Kurian et al. 2016). Non-genetic BC risk factors include hormonal BC risk factors (e.g. use of hormone-replacement therapy, oral contraception), reproductive BC risk factors (e.g. age of first pregnancy, breastfeeding, age at menarche, age at menopause) and lifestyle BC risk factors (e.g. obesity, physical activity, alcohol consumption) (Harvie et al. 2015).
Several models and tools have been developed to enable healthcare professionals to assess the probability of developing BC and/or of carrying a deleterious mutation. These models account for the individual or combined effects of different BC risk factors (Antoniou et al. 2008; Amir et al. 2010; Meads et al. 2012; Quante et al. 2012; Kurian et al. 2016; Cintolo-Gonzalez et al. 2017). They can be used in clinical practice to appraise needs for referral for genetic counselling and testing and to inform decisions on risk management options such as enhanced screening, risk-reducing surgical interventions or chemoprevention (Padamsee et al. 2017). These models differ according to the specific BC risk factors they incorporate, their developmental method and clinical applications.
We report the results of a survey performed as part of the BRIDGES research program (http://cordis.europa.eu/project/rcn/193315_en.html) that aims to inform the development and implementation of a comprehensive genetic test in BC risk assessment and to combine this with additional non-genetic BC risk factors. The latter will be achieved through further development of the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA) model and BOADICEA Web Application (BWA) (Lee et al. 2014, 2016).
Many BC risk assessment tools are currently available. However, it is unclear which clinicians use them, why they use them and whether these tools fulfil their requirements. In particular, we wished to assess the clinical acceptability of the BOADICEA model and the BWA. In a previous article (Bredart et al. 2018), we reported on clinicians’ perceptions of the BOADICEA model and BWA v3 tool in terms of usability (e.g., data entry timing, risk communication format). In this study, we explored the relationship between the perceived importance attributed to BC risk factors by professional profile, in relation to respondents’ knowledge and usage frequencies of the BWA as well as of six other BC risk assessment tools commonly used in family cancer clinics. The results of our analyses will help to identify perceived deficiencies in the BWA and guide further software development, so that the next version of the tool will enable more comprehensive BC risk assessment in genetics and oncology practice.
Methods
This was a cross-sectional study based on an international survey.
We conducted an online survey (Bredart et al. 2018) involving one reminder using the LimeSurvey application (http://www.limesurvey.org) (LimeSurvey Project Team, Carsten Schmitz 2015) during May to September 2016.
The survey targeted clinicians who were among the potential 7500 individuals who registered to use the BWA since 2007. In addition, BRIDGES investigators were asked to contact members of their national genetics societies (NGS) to invite them to complete the survey (if they had not already responded through the BOADICEA website). A total of 225, 170, 37 and 32 individuals were contacted from the British, French, Dutch and Swedish NGS, respectively.
Survey development
The survey questions addressed the following: (1) practice in genetic counselling and testing for cancer predisposition; (2) importance attributed to BC familial, hormonal, reproductive and lifestyle BC risk factors; (3) knowledge/usage frequency of the Gail (Gail et al. 1989), Claus (Claus et al. 1991; Parmigiani et al. 2007), Myriad (Shattuck-Eidens et al. 1997; Frank et al. 2002), BRCAPRO (Parmigiani et al. 1998; Mazzola et al. 2015), IBIS (International Breast Cancer Study) (Tyrer et al. 2004; Quante et al. 2012; Brentnall et al. 2015; Cuzick et al. 2016) and BWA (Antoniou et al. 2004, 2008; Cunningham et al. 2012; MacInnis et al. 2013; Lee et al. 2014, 2016) BC risk assessment tools, and Manchester Scoring System (MSS) (Evans et al. 2004, 2005) and data entry timing using these tools; and (4) socio-demographic and professional background (Supplementary material A).
The questionnaire was developed in line with BRIDGES program objectives, which specified an assessment of the acceptability of the BOADICEA model and the BWA in clinical practice. As the next BWA update is expected to integrate non-genetic and additional genetic BC risk factors, we assessed the degree of importance attributed to familial, hormonal, reproductive and lifestyle risk factors to BC risk assessment (Harvie et al. 2015). For each BC risk factor, five response options from least to most important were provided (see Table 4).
We assessed BWA use with that of six other BC risk assessment tools that could be used in family cancer clinics, implementing the Gail (Gail et al. 1989), Claus (Claus et al. 1991; Parmigiani et al. 2007), Myriad (Shattuck-Eidens et al. 1997; Frank et al. 2002), BRCAPRO (Parmigiani et al. 1998; Mazzola et al. 2015) or IBIS (Tyrer et al. 2004; Quante et al. 2012; Brentnall et al. 2015; Cuzick et al. 2016) models or MSS (Evans et al. 2004, 2005).
These models focus primarily on family/personal cancer history. Their distinctive features are briefly summarised below and described in a table adapted from Kurian et al. (2016) and Cintolo-Gonzalez et al. (2017) (Table 1). The Gail model (or NCI Breast Cancer Risk Assessment Tool: BCRAT) (Gail et al. 1989) and Claus model (Claus et al. 1991; Parmigiani et al. 2007) are used to compute BC risk (only Gail incorporates both reproductive and familial BC risk factors). The MSS (Evans et al. 2004, 2005) and Myriad I or II models (Shattuck-Eidens et al. 1997; Frank et al. 2002) are used to compute the probability of carrying a deleterious BRCA1 and/or BRCA2 mutation based on cancer family history. In addition to familial/personal BC risk factors, the MSS also accounts for the presence of a BRCA1 or BRCA2 mutation, breast cancer pathology and cancers other than breast or ovarian. The BRCAPRO (Parmigiani et al. 1998; Mazzola et al. 2015), IBIS (Tyrer et al. 2004; Quante et al. 2012; Brentnall et al. 2015; Cuzick et al. 2016) and BOADICEA (Antoniou et al. 2004, 2008; Cunningham et al. 2012; MacInnis et al. 2013; Lee et al. 2014, 2016) models predict BRCA1 or BRCA2 mutation carrier probability and BC risk. In addition, BRCAPRO and BOADICEA predict contralateral breast and ovarian cancer risk (Cintolo-Gonzalez et al. 2017). BRCAPRO incorporates family history, BRCA1 or BRCA2 mutation status, molecular markers, mastectomy/oophorectomy and ethnicity factors. IBIS incorporates not only family history, BRCA1 or BRCA2 mutation status and breast cancer pathology but also reproductive, hormone replacement therapy (HRT) and body mass index (BMI) BC risk factors. The BOADICEA model allows for extensive family history; tumour pathology such as oestrogen and progesterone receptors and HER2, CK5/6 and CK14 status of BC in family member(s) to be taken into account (Lee et al. 2014). BOADICEA also includes the effects of truncating mutations in PALB2, CHEK2 and ATM (Lee et al. 2016).
For all seven BC risk assessment tools, we assessed knowledge (‘I don’t know the model’), use frequency on a five-point Likert scale ranging from never to always (see Table 3) and convenience (i.e. the time required for data entry).
A preliminary version of the overall survey was designed following survey method recommendations (Edwards et al. 2009; Cottrell et al. 2015). It was pilot-tested with clinical geneticists, genetic counsellors, gynaecologists, psycho-oncologists, a radiotherapist and a methodologist (n = 21).
Data analysis
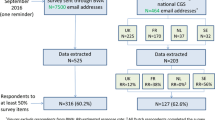
As shown in Fig. 1, 525 and 203 respondents’ data were extracted from the BOADICEA website and NGS survey sources respectively. The response rate obtained from the BOADICEA website survey source could not be estimated as the survey was sent to registered individuals who might no longer use the tool, and tracking the tool use is not legally permitted. Among respondents contacted through NGS, the response rate was 43.7% (203 respondents out of 464 NGS members, excluding those who responded through the BOADICEA website).
Based on psychometric analyses (Husson et al. 2011; Jolliffe 2002), we developed indicators of genetic clinical activity level (i.e. one scale comprising six survey items), and of importance attributed to BC risk factors (i.e. five single items addressing familial and personal cancer history, breast tumour pathology, BMI and breastfeeding), and three multi-item scales corresponding to hormonal BC risk factors (i.e. oral contraception, HRT and exogenous hormone consumption), reproductive BC risk factors (i.e. age at first menstrual period, age at menopause and child bearing at younger age), and lifestyle BC risk factors (i.e. alcohol consumption, smoking and physical activity).
Knowledge and usage frequency were reported in percentages. We computed the number of respondents by the number of BC risk assessment tools used at least occasionally or regularly. Correlation coefficients were calculated between the usage frequencies of the different BC risk assessment tools. We compared the degree of importance attributed to each BC risk factor by health profession (i.e. clinical geneticists, genetic counsellors or nurses and oncology specialists) using a chi-squared test.
Logistic multiple regression analyses (Tabachnick and Fidell 2013) were performed on dichotomized variables addressing knowledge and usage frequency of six of the BC risk assessment tools (excluding Myriad which received less than 100 responses) comparing ignorance of the tool or absent use versus regular or more frequent use.
We explored clinicians’ background characteristics and the degree of importance that they attributed to different BC risk factors as potential explanatory variables, controlling for gender. Age was significantly correlated to respondents’ clinical seniority, so to maintain parsimony, it was not included as an explanatory variable.
In further multivariate analyses, we tested the effect of data entry time for each BC risk assessment tool, including only respondents who use the respective tools. The logarithm of data entry time was computed to allow a normal data distribution.
Statistical analyses were performed with R software version 3.3.1 (R Core Team 2016).
Results
Overall, 443 respondents completed at least 50% of the survey, comprising 316 (71.3%) and 127 (28.7%) via the BOADICEA website and NGS survey sources respectively (Fig. 1). Due to missing responses, especially in the last sections of the questionnaire, less data on socio-demographics and professional characteristics are available (N = 394).
Sample characteristics
As shown in Table 2, a wide range of geographical regions/countries were represented among respondents, including Southern Europe (28%), Western Europe (21%), UK (20%), North America (18%), Australia (8%) and other countries (6%). Respondents also varied in terms of age and years of clinical experience but were mainly female (77%); most had received a specific training in cancer genetic counselling and testing (70%) and had a clinical activity of 5 to 10 patients per week (42%).
Breast cancer risk assessment tool knowledge and usage frequency
The proportion of participants who did not know one of the BC risk assessment tools included in the survey (excluding the BWA which was reported as not known by two respondents) ranged from 24 (5%) (BRCAPRO) to 61 (14%) (Myriad).
The BWA was used at least occasionally by 413 survey participants (93%) (Table 3). The Gail, Claus, MSS, Myriad, BRCAPRO and IBIS tools were used at least occasionally by 25, 27, 37, 13, 30 and 41% respondents, respectively.
Regarding the number of tools that were used, the most common observation was the use of one tool at least regularly, by 36% of respondents. The usage frequency of the BWA and other tools was uncorrelated; only the usage frequency of Gail was correlated to the use other tools, i.e. Myriad (r = 0.51), Claus (r = 0.45), BRCAPRO (r = 0.44) and IBIS (r = 0.41) (data not shown).
Breast cancer risk assessment tool data completion time
Over the different BC risk assessment tools, the mean (standard deviation) time in minutes to input clinical data ranged from 3.6 (3.6) (MSS) to 15.6 (10.8) (BWA) (Supplementary material B).
Perceived importance of breast cancer risk factors
To estimate BC risk, cancer family history was perceived as most important by most respondents (72%). Non-genetic BC risk factors were perceived as most important by between 1.3% (breastfeeding, oral contraception) and 5.4% (age at first menstrual period) of respondents (Table 4).
Familial history of cancer was perceived as most important significantly more by geneticists than oncology health professionals (p ≤ 0.01). More genetic counsellors/nurses than other health professionals considered breast tumour pathology as most important (p ≤ 0.01) (Tables 4 and 5).
Oncology health professionals attributed higher importance to the following BC risk factors than genetics health professionals: breastfeeding (p ≤ 0.05), alcohol consumption (p ≤ 0.0001), oral contraception (p ≤ 0.01), HRT (p ≤ 0.0001) and physical exercise (p ≤ 0.01).
Predictors of breast cancer risk assessment tool usage frequency
The professional background characteristics were not associated with the frequency of use of BRCAPRO or the BWA.
Genetic counsellors/nurses and oncology health professionals used the following tools more frequently than clinical geneticists: Gail (OR [CI] = 12.30 [3.17–47.9]; OR [CI] = 13.60 [2.82–65.2]) and IBIS (OR [CI] = 8.41 [3.39–20.8]; OR [CI] = 5.47 [1.79–16.6]). In addition, genetic counsellors/nurses used the MSS more frequently than clinical geneticists (OR [CI] = 3.22, [1.64–6.27]). Longer clinical experience was associated with a more frequent use of the Gail (OR [CI] = 2.97 [1.01–8.79]), Claus (OR [CI] = 3.06 [1.23–7.54]) and IBIS (OR [CI] = 2.61 [1.12–6.10]) tools. The level of clinical activity and specific cancer genetic counselling and testing training was not associated with the use of any of these tools.
To estimate BC risk, respondents who attributed less importance to personal BC (OR [CI] = 0.49 [0.28–0.86]) and more importance to breast tumour pathology (OR [CI] = 3.90 [1.90–7.97]) used the BWA more frequently; the reverse was (unexpectedly) observed for the Gail (personal BC history: OR [CI] = 2.66 [1.10–6.46]; breast tumour pathology: OR [CI] = 0.21 [0.07–0.62]) and Claus (personal BC history: OR [CI] = 2.56 [1.24–5.26]; breast tumour pathology: OR [CI] = 0.41 [0.18–0.92]) tools. The use of the BWA was (unexpectedly) associated with the importance given to hormonal (exogenous) factors (OR [CI] = 1.77 [1.20–2.60]). Considering reproductive BC factors as important was associated with more frequent use of the Gail (OR [CI] = 3.06 [1.67–5.65]), Claus (OR [CI] = 1.88 [1.13–3.11]) and IBIS (OR [CI] = 4.81 [2.86–8.14]) tools and less frequent use of the MSS (OR [CI] = 0.57 [0.38–0.86]). IBIS was used less frequently by individuals who considered breastfeeding as important (OR [CI] = 0.53 [0.33–0.86]).
Among non-genetic BC risk factors, the importance attributed to lifestyle and BMI risk factors to estimate BC risk was not associated with the use of any of these tools.
Discussion
Respondents to this survey were mostly BWA users (93%), and few were unaware of other BC risk assessment tools commonly used in cancer genetics clinics. Thus, results presented here describe factors that potentially influence the respondents’ choice of using the BWA or another tool.
Uptake of a BC risk assessment tool may depend on its perceived validity, which is related to the type and number of BC risk factors incorporated within the underlying statistical model and any accompanying validation studies. So, in line with the characteristics of the BC risk assessment tools (described in Table 1), respondents who attributed more importance to breast tumour pathology risk factors used the BWA more frequently and used the Gail and Claus tools less frequently. Similarly, those who attributed more importance to reproductive factors used the Gail and IBIS tools more frequently. However, the relationship between some BC risk factors considered important by our respondents and the more frequent use of some tools was unexpected, given that some tools do not include the BC risk factors considered important by their users (perhaps suggesting lack of understanding of the underlying risk model). For example, we found that the use of the BWA was related to the importance attributed to exogenous hormonal BC factors even though this model does not take these BC risk factors into account. In this study, most respondents regularly used only one tool. It is possible that one tool is selected to optimally respond to one’s major clinical needs. For example, the BWA may be used, in spite of not currently accounting for hormonal factors, because it considers a number of familial and genetic factors.
Uptake of a BC risk assessment tool may also depend on the health professional’s patient population and role (i.e. to guide referral to family history clinics, to assess BC risk or the probability of harbouring a deleterious mutation, to make oncology treatment decisions, to advise on cancer risk management). Respondents’ professional background appeared related to the importance attributed to familial or hormonal, reproductive and lifestyle factors. This may explain the distinct uptake of some BC risk assessment tools according to the type of respondents’ profession. Indeed, oncology specialists attributed more importance to hormonal and lifestyle factors and also used the Gail and IBIS tools more frequently than clinical geneticists. However, genetic counsellors and nurses also used these tools more frequently as well as the MSS, which may be related to role sharing between medical and non-medical genetics clinicians. BWA use was not related to the type of health profession or the use of other tools, suggesting that this tool may respond to various clinical needs.
In contrast, the Gail tool was moderately associated with the use of other tools such as Claus, Myriad, BRCAPRO and IBIS. In fact, our respondents were mostly genetics clinicians, and validation studies suggest that Gail is less appropriate to assess BC risk in hereditary high-risk populations [Evans and Howell 2007]. Moreover, Gail does not predict mutation carrier status, so this tool seems to require a complementary assessment tool for professionals in this survey.
Uptake of BC risk assessment tools may be related to usability and ease of data entry (e.g. depending on the amount of information) and accessibility (i.e. availability on the Web and ease with which statistical outputs can be understood and communicated to patients). In this study, only the uptake of Claus and the MSS was related to the usability of the tool (data presented in supplementary material C). In cancer genetics clinics, these tools may be used systematically for all patients to appraise the need for genetic testing and so they must be easy to use. In contrast, it is possible that tools with lower usability (that require more time to enter data) such as the BWA may be acceptable in specific situations, e.g. to refine a BC risk assessment or estimate the risk of contralateral BC and other cancers.
The importance given to lifestyle and BMI BC risk factors was not associated with the use of any tool. This was expected as the BC risks associated with these risk factors are not as high as those associated with genetic risks (Amir et al. 2010; Kurian et al. 2016). Moreover, IBIS was the only tool that takes BMI into account in risk calculations. The presence of a substantial family history of BC and the likelihood of carrying a deleterious mutation are important when predicting the incidence of BC, and thus are the focus of genetics clinicians. However, BMI (Quante et al. 2015; Lecarpentier et al. 2015) and reproductive and hormonal BC risk factors (Lecarpentier et al. 2015) seem to modify the BC risk associated with high-risk genetic variants. Moreover, the influence of lifestyle on BC risk has also been suggested among women at increased risk or with a BRCA1/2 gene mutation (Easton et al. 2015). Lifestyle factors may be targeted for change by health interventions to reduce BC risk (Meads et al. 2012). Studies have shown that discussion of these factors with counselees is variable across health professional specialties (Julian-Reynier et al. 2015) and may be infrequent in clinical genetics practice (Albada et al. 2014). As the next version of the BWA will incorporate these BC risk factors, this updated tool may facilitate more comprehensive BC risk counselling.
Our study has several limitations:
-
1.
This survey was conducted via the BOADICEA website and European NGS, and so the respondent sample mainly comprised BWA users and European genetics clinicians. Thus, our findings may not be generalised to other, more diverse clinician populations in other geographic areas. In Canada, for example, general practitioners, specialists and genetic counsellors most frequently used the BRCAPRO and Gail tools, whereas the BWA was less frequently used (by 13% compared to 25 and 18% for the BRCAPRO and Gail, respectively) (Amara et al. 2015).
-
2.
Some BC risk assessment tools investigated were used by a limited number of respondents, and so we may have lacked power to provide reliable estimates of the BC risk factors related to their use. In addition, the overall high usage frequency of BWA in this sample and thus the lack of variation in this tool usage frequency may have also hampered the detection of some relationships. For example, although some experience is needed for proper use of the BWA, more frequent use of some of the investigated tools (but not BWA) was related to increased clinicians’ seniority.
-
3.
The survey design allows us to comment on the associations, but we cannot ascertain the directionality of the relationships, nor their specificity over time, along stages of the BC genetic counselling process.
Conclusion
Our study provides novel insights into the acceptability of the BWA by analysis of BC risk factors incorporated in this tool and other common BC risk assessment tools. These results suggest that while the BWA does fulfil various clinicians’ needs, it does not incorporate some BC risk factors considered important by its users. The results of our analyses will help to identify perceived deficiencies in the BWA and guide further software development (including integration of additional genetic and non-genetic BC risk factors), so that the next version of the BWA will facilitate more comprehensive BC risk assessment in genetics and oncology practice.
Change history
22 July 2019
The published online version contains mistake in author list. The correct presentation of the name Rita Schmutlzer is <Emphasis Type="Bold">Rita Schmutzler.</Emphasis>
References
Albada A, Vernooij M, van Osch L, Pijpe A, van Dulmen S, Ausems MG (2014) Does and should breast cancer genetic counselling include lifestyle advice? Familial Cancer 13:35–44
Amara N, Blouin-Bougie J, Jbilou J, Halilem N, Simard J, Landry R (2015) The knowledge value-chain of genetic counseling for breast cancer: an empirical assessment of prediction and communication processes. Familial Cancer 15(1):1–17
Amir E, Freedman OC, Seruga B, Evans DG (2010) Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst 102:680–691
Antoniou AC, Pharoah PP, Smith P, Easton DF (2004) The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer 91:1580–1590
Antoniou AC, Hardy R, Walker L, Evans DG, Shenton A, Eeles R, Shanley S, Pichert G, Izatt L, Rose S, Douglas F, Eccles D, Morrison PJ, Scott J, Zimmern RL, Easton DF, Pharoah PD (2008) Predicting the likelihood of carrying a BRCA1 or BRCA2 mutation: validation of BOADICEA, BRCAPRO, IBIS, Myriad and the Manchester scoring system using data from UK genetics clinics. J Med Genet 45:425–431
Bredart A, Kop JL, Antoniou AC, Cunningham AP, De Pauw A, Tischkowitz M, Ehrencrona H, Dolbeault S, Robieux L, Rhiem K, Easton DF, Devilee P, Stoppa-Lyonnet D, Schmutlzer R (2018) Use of the BOADICEA web application in clinical practice: appraisals by clinicians from various countries. Familial Cancer 17(1): 31−41
Brentnall AR, Harkness EF, Astley SM, Donnelly LS, Stavrinos P, Sampson S, Fox L, Sergeant JC, Harvie MN, Wilson M, Beetles U, Gadde S, Lim Y, Jain A, Bundred S, Barr N, Reece V, Howell A, Cuzick J, Evans DG (2015) Mammographic density adds accuracy to both the Tyrer-Cuzick and Gail breast cancer risk models in a prospective UK screening cohort. Breast Cancer Res 17(1):147
Cintolo-Gonzalez JA, Braun D, Blackford AL, Mazzola E, Acar A, Plichta JK, Griffin M, Hughes KS (2017) Breast cancer risk models: a comprehensive overview of existing models, validation, and clinical applications. Breast Cancer Res Treat 164(2):263–284
Claus EB, Risch N, Thompson WD (1991) Genetic analysis of breast cancer in the cancer and steroid hormone study. Am J Hum Genet 48:232–242
Cottrell E, Roddy E, Rathod T, Thomas E, Porcheret M, Foster NE (2015) Maximising response from GPs to questionnaire surveys: do length or incentives make a difference? BMC Med Res Methodol 15:3
Couch FJ, Nathanson KL, Offit K (2014) Two decades after BRCA: setting paradigms in personalized cancer care and prevention. Science 343:1466–1470
Cunningham AP, Antoniou AC, Easton DF (2012) Clinical software development for the web: lessons learned from the BOADICEA project. BMC Med Inform Decis Mak 12:30
Cuzick J, Brentnall AR, Segal C, Byers H, Reuter C, Detre S, Lopez-Knowles E, Sestak I, Howell A, Powles TJ, Newman WG, Dowsett M (2016) Impact of a panel of 88 single nucleotide polymorphisms on the risk of breast cancer in high-risk women: results from two randomized tamoxifen prevention trials. J Clin Oncol 35(7):743–750
DeSantis CE, Bray F, Ferlay J, Lortet-Tieulent J, Anderson BO, Jemal A (2015) International variation in female breast cancer incidence and mortality rates. Cancer Epidemiol Biomark Prev 24:1495–1506
Easton DF, Pharoah PD, Antoniou AC, Tischkowitz M, Tavtigian SV, Nathanson KL, Devilee P, Meindl A, Couch FJ, Southey M, Goldgar DE, Evans DG, Chenevix-Trench G, Rahman N, Robson M, Domchek SM, Foulkes WD (2015) Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med 372:2243–2257
Edwards PJ, Roberts I, Clarke MJ, Diguiseppi C, Wentz R, Kwan I, Cooper R, Felix LM, Pratap S (2009) Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev 8(3):MR000008
Evans DG, Eccles DM, Rahman N, Young K, Bulman M, Amir E, Shenton A, Howell A, Lalloo F (2004) A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J Med Genet 41:474–480
Evans DG, Lalloo F, Wallace A, Rahman N (2005) Update on the Manchester scoring system for BRCA1 and BRCA2 testing. J Med Genet 42(7):e39
Evans DG, Howell A (2007) Breast cancer risk-assessment models. Breast Cancer Res 9(5): 213
Frank TS, Deffenbaugh AM, Reid JE, Hulick M, Ward BE, Lingenfelter B, Gumpper KL, Scholl T, Tavtigian SV, Pruss DR, Critchfield GC (2002) Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol 20:1480–1490
Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, Mulvihill JJ (1989) Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 81:1879–1886
Harvie M, Howell A, Evans DG (2015) Can diet and lifestyle prevent breast cancer: what is the evidence? Am Soc Clin Oncol Educ Book 35:e66–e73
Husson F, Lê S, Pagès J (2011) Exploratory multivariate analysis by example using R. CRC Press, Boca Raton
Jolliffe IT (2002) Principal component analysis. Springer, Second Edition
Julian-Reynier C, Bouhnik AD, Evans DG, Harris H, van Asperen CJ, Tibben A, Schmidtke J, Nippert I (2015) General practitioners and breast surgeons in France, Germany, Netherlands and the UK show variable breast cancer risk communication profiles. BMC Cancer 15:243
Kurian AW, Antoniou AC, Domchek SM (2016) Refining breast cancer risk stratification: additional genes, additional information. Am Soc Clin Oncol Educ Book 35:44–56
Lecarpentier J, Nogues C, Mouret-Fourme E, Buecher B, Gauthier-Villars M, Stoppa-Lyonnet D, Bonadona V, Fricker JP, Berthet P, Caron O, Coupier I, Pujol P, Faivre L, Gesta P, Eisinger F, Mari V, Gladieff L, Lortholary A, Luporsi E, Leroux D, Venat-Bouvet L, Maugard CM, Colas C, Tinat J, Lasset C, Andrieu N (2015) Breast cancer risk associated with estrogen exposure and truncating mutation location in BRCA1/2 carriers. Cancer Epidemiol Biomark Prev 24:698–707
Lee AJ, Cunningham AP, Kuchenbaecker KB, Mavaddat N, Easton DF, Antoniou AC (2014) BOADICEA breast cancer risk prediction model: updates to cancer incidences, tumour pathology and web interface. Br J Cancer 110:535–545
Lee AJ, Cunningham AP, Tischkowitz M, Simard J, Pharoah PD, Easton DF, Antoniou AC (2016) Incorporating truncating variants in PALB2, CHEK2, and ATM into the BOADICEA breast cancer risk model. Genet Med 18:1190–1198
LimeSurvey Project Team, Carsten Schmitz (2015). LimeSurvey: an open source survey tool. LimeSurvey Project Hamburg, Germany. URL http://www.limesurvey.org
MacInnis RJ, Bickerstaffe A, Apicella C, Dite GS, Dowty JG, Aujard K, Phillips KA, Weideman P, Lee A, Terry MB, Giles GG, Southey MC, Antoniou AC, Hopper JL (2013) Prospective validation of the breast cancer risk prediction model BOADICEA and a batch-mode version BOADICEACentre. Br J Cancer 109:1296–1301
Mazzola E, Blackford A, Parmigiani G, Biswas S (2015) Recent enhancements to the genetic risk prediction model BRCAPRO. Cancer Inform 14:147–157
Meads C, Ahmed I, Riley RD (2012) A systematic review of breast cancer incidence risk prediction models with meta-analysis of their performance. Breast Cancer Res Treat 132:365–377
Padamsee TJ, Wills CE, Yee LD, Paskett ED (2017) Decision making for breast cancer prevention among women at elevated risk. Breast Cancer Res 19:34
Parmigiani G, Berry D, Aguilar O (1998) Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet 62:145–158
Parmigiani G, Chen S, Iversen ES Jr, Friebel TM, Finkelstein DM, Anton-Culver H, Ziogas A, Weber BL, Eisen A, Malone KE, Daling JR, Hsu L, Ostrander EA, Peterson LE, Schildkraut JM, Isaacs C, Corio C, Leondaridis L, Tomlinson G, Amos CI, Strong LC, Berry DA, Weitzel JN, Sand S, Dutson D, Kerber R, Peshkin BN, Euhus DM (2007) Validity of models for predicting BRCA1 and BRCA2 mutations. Ann Intern Med 147:441–450
Quante AS, Whittemore AS, Shriver T, Strauch K, Terry MB (2012) Breast cancer risk assessment across the risk continuum: genetic and nongenetic risk factors contributing to differential model performance. Breast Cancer Res 14(6):R144
Quante AS, Herz J, Whittemore AS, Fischer C, Strauch K, Terry MB (2015) Assessing absolute changes in breast cancer risk due to modifiable risk factors. Breast Cancer Res Treat 152:193–197
R Core Team (2016) R: a language and environment for statistical computing. In: R Foundation for Statistical Computing, Vienna, Austria URL https://www.R-project.org/
Shattuck-Eidens D, Oliphant A, McClure M, McBride C, Gupte J, Rubano T, Pruss D, Tavtigian SV, Teng DH, Adey N, Staebell M, Gumpper K, Lundstrom R, Hulick M, Kelly M, Holmen J, Lingenfelter B, Manley S, Fujimura F, Luce M, Ward B, Cannon-Albright L, Steele L, Offit K, Thomas A et al (1997) BRCA1 sequence analysis in women at high risk for susceptibility mutations. Risk factor analysis and implications for genetic testing. JAMA 278:1242–1250
Tabachnick BG, Fidell LS (2013) Using multivariate statistics, 6th edn. Pearson, Boston
Tyrer J, Duffy SW, Cuzick J (2004) A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 23:1111–1130
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Study participants consented informed to complete an online questionnaire through LimeSurvey. (Project Team, Carsten Schmitz (2015). LimeSurvey: an open source survey tool. LimeSurvey Project Hamburg, Germany. URL http://www.limesurvey.org).
Electronic supplementary material
ESM 1
(DOC 109 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Brédart, A., Kop, JL., Antoniou, A.C. et al. Clinicians’ use of breast cancer risk assessment tools according to their perceived importance of breast cancer risk factors: an international survey. J Community Genet 10, 61–71 (2019). https://doi.org/10.1007/s12687-018-0362-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12687-018-0362-8