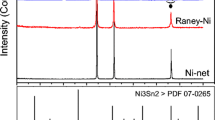
Abstract
Alkaline water electrolysis is a versatile technology for hydrogen production in situ. In this work, we study two different electrodeposited Ni-Mo alloys, with good electrocatalytic activity to hydrogen evolution reaction (HER). We propose Ni as substrate avoiding the corrosion and electrolyte contamination problem derived from eventual coating failure during long-term operations. The stable behavior of Ni as substrate as well as its known HER catalytic activity in alkaline electrolyzers make it attractive as a good option for our purpose. We prepare and characterize several Ni-Mo coatings over Ni by direct and pulse current supply electrodepositions. Ni-Mo coating prepared by pulse plating with a peak current density of 280 mA·cm−2 and 35% duty cycle exhibited the best electrocatalytic activity for HER. The coating is an amorphous alloy whose XRD peaks correspond to an f.c.c. Ni structure, with Mo substituent atoms and a rough cauliflower-like microstructure, with Mo content of 23 at.%. After 1000 cyclic voltammetry cycles, the electrocatalytic activity of the Ni-Mo electrocatalyst remains practically unchanged, and during 200 h of H2 evolution it maintains an overvoltage reduction of ca. 200 mV compared with bare Ni.

ᅟ













Similar content being viewed by others
References
M. Wang, Z. Wang, X. Gong, Z. Guo, The intensification technologies to water electrolysis for hydrogen production – a review. Renew. Sust. Energ. Rev. 29, 573–588 (2014)
M. Kraglund, D. Aili, K. Jankova, E. Christensen, Q. Li, J.O. Jensen, Zero-gap alkaline water electrolysis using ion-solvating polymer electrolyte membranes at reduced KOH concentrations. J. Electrochem. Soc. 163(11), F3125–F3131 (2016)
D. Pletcher, X. Li, Prospects for alkaline zero gap water electrolysers for hydrogen production. Int. J. Hydrog. Energy 36(23), 15089–15104 (2011)
F. Safizadeh, E. Ghali, G. Houlachi, Electrocatalysis developments for hydrogen evolution reaction in alkaline solutions. A review. Int. J. Hydrog. Energy 40, 256 (2015)
M. Giz, S. Bento, E. Gonzalez, NiFeZn codeposit as a cathode material for the production of hydrogen by water electrolysis. Int. J. Hydrog. Energy 25(7), 621–626 (2000)
C. Hu, C. Tsay, A. Bai, Optimization of the hydrogen evolution activity on zinc/nickel deposits using experimental strategies. Electrochim. Acta 48(7), 907–918 (2003)
R. Solmaz, G. Kardas, Hydrogen evolution and corrosion performance of NiZn coatings. Energy Convers. Manag. 48(2), 583–591 (2007)
J. Cai, J. Xu, J. Wanga, L. Zhang, H. Zhou, Y. Zhong, D. Chen, H. Fan, H. Shao, J. Zhang, C. Cao, Fabrication of three-dimensional nanoporous nickel films with tunable nanoporosity and, their excellent electrocatalytic activities for hydrogen evolution reaction. Int. J. Hydrog. Energy 38(2), 934–941 (2013)
L. Vázquez-Gómez, S. Cattarin, P. Guerriero, M. Musiani, Influence of deposition current density on the composition and properties of electrodeposited Ni + RuO2 and Ni + IrO2 composites. J. Electroanal. Chem. 634(1), 42–48 (2009)
S. Shibli, J. Sebeelamol, Development of Fe2O3 - TiO2 mixed oxide incorporated NiP coating for electrocatalytic hydrogen evolution reaction. Int. J. Hydrog. Energy 38, 227 (2013)
C. Xu, J. Zhou, M. Zeng, X. Fu, X. Liu, J. Li, Electrodeposition mechanism and characterization of Ni-Mo alloy and its electrocatalytic performance for hydrogen evolution. Int. J. Hydrog. Energy 41(31), 13341–13349 (2016)
I. McKay, J. Schwalbe, E. Goodman, J. Willis, A. Majumdar, M. Cargnello, Elucidating the synergistic mechanism of nickel–molybdenum electrocatalysts for the hydrogen evolution reaction. MRS Commun. 6(03), 241–246 (2016)
G. Temam, H. Temam, S. Benramache, Surface morphology and electrochemical characterization of electrodeposited Ni-Mo nanocomposites as cathodes for hydrogen evolution. Chinese Phys. B 24(10), 108202–108201 (2015)
O. Aaboubi, A.Y. Ali Omar, A. Franczak, K. Msellak, Investigation of the electrodeposition kinetics of Ni–Mo alloys in the presence of magnetic field. J. Electroanal. Chem. 737, 226–234 (2015)
M. Wang, Z. Wang, X. Yu, Z. Guo, Facile one-step electrodeposition preparation of porous Ni-Mo film as electrocatalyst for hydrogen evolution reaction. Int. J. Hydrog. Energy 40(5), 2173–2181 (2015)
J.Y. Huot, M.L. Trudeau, R. Schulz, Low hydrogen overpotential nanocrystalline Ni-Mo cathodes for alkaline water electrolysis. J. Electrochem. Soc. 138(5), 1316 (1991)
P. Kedzierzawski, D. Oleszak, M. Janik-Czachor, Hydrogen evolution on hot and cold consolidated Ni–Mo alloys produced by mechanical alloying. Mater. Sci. Eng. A300, 105 (2001)
R. Schulz, J.Y. Huot, M.L. Trudeau, L. Dignard-Bailey, Z.H. Yan, S. Jin, A. Lamarre, E. Ghali, A. Van Neste, Nanocrystalline Ni-Mo alloys and their application in electrocatalysis. J. Mater. Res. 9(11), 2998–3008 (1994)
E.K. Athanassiou, R.N. Grass, N. Osterwalder, W.J. Stark, Preparation of homogeneous, bulk nanocrystalline Ni/Mo alloys with tripled Vickers hardness using flame-made metal nanoparticles. Chem. Mater. 19(20), 4847–4854 (2007)
G. Schiller, R. Henne, P. Mohr, P. Peinecke, High performance electrodes for an advanced intermittently operated 10-kW alkaline water electrolyzer. Int. J. Hydrog. Energy 23(9), 761–765 (1998)
L. Birry, A. Lasia, Studies of the hydrogen evolution reaction on Raney nickel-molybdenum electrodes. J. Appl. Electrochem. 34(7), 735–749 (2004)
M.R. Gennero de Chialvo, A.C. Chialvo, Hydrogen evolution reaction on smooth Ni (1-x) + Mo (x) alloys (0 ≤ x ≤ 0.25). J. Electroanal. Chem. 448, 87 (1998)
L. Xiao, S. Zhang, J. Pan, C. Yang, M. He, L. Zhuang, J. Lu, First implementation of alkaline polymer electrolyte water electrolysis working only with pure water. Energy Environ. Sci. 5, 7869 (2012)
X. Yu, M. Wang, Z. Wang, X. Gong, Z. Guo, Electrodeposited under super gravity field as electrocatalyst for hydrogen evolution reaction. J. Phys. Chem. C 121(31), 16792–16802 (2017)
E. Navarro-Flores, Z. Chong, S. Omanovic, Characterization of Ni, Ni-Mo, NiW and NiFe electroactive coatings as electrocatalysts for hydrogen evolution in an acidic medium. J. Mol. Catal. A Chem. 226, 179 (2005)
Q. Han, S. Cui, N. Pu, J. Chen, K. Liu, X. Wei, A study on pulse plating amorphous Ni-Mo alloy coating used as HER cathode in alkaline medium. Int. J. Hydrog. Energy 35(11), 5194–5201 (2010)
W. Hu, Electrocatalytic properties of new electrocatalysts for hydrogen evolution in alkaline water electrolysis. Int. J. Hydrog. Energy 25(2), 111–118 (2000)
J.O.’.M. Bockris, A. Damjanovic, R.J. Mannan, Catalysis of the electrodic hydrogen evolution and dissolution reactions on rationally chosen substrates. J. Electroanal. Chem. 18(4), 349–361 (1968)
T. Watanabe, Nano Plating, Microstructure Control Theory of Plated Films and Data Base of Plated Film Microstructure, Elsevier, ISBN 0–08-0440193 (2004)
S. Nakahara, R. Weil, Initial stages of electro-mono-crystallization of nickel on copper-film substrates. J. Electrochem. Soc. 120(11), 1462 (1973)
N. Krstajic, M. Popovic, B. Grgur, M. Vojnovic, D. Sepa, On the kinetics of the hydrogen evolution reaction on nickel in alkaline solution, Part I. The mechanism. J. Electroanal. Chem. 512(1-2), 16–26 (2001)
E.A. Franceschini, G.I. Lacconi, H.R. Corti, Kinetics of the hydrogen evolution on nickel in alkaline solution: new insight from rotating disk electrode and impedance spectroscopy analysis. Electrochim. Acta 159, 210–218 (2015)
J.M. Jaksic, M.V. Vojnovic, N.V. Krstajic, Kinetic analysis of hydrogen evolution at Ni–Mo alloy electrodes. Electrochim. Acta 45(25-26), 4151–4158 (2000)
J. van Drunen, B.K. Pilapil, Y. Makonnen, D. Beauchemin, B.D. Gates, G. Jerkiewicz, Electrochemically active nickel foams as support materials for nanoscopic platinum electrocatalysts. ACS Appl. Mater. Interfaces 6, 12046 (2014)
A. Seghiouer, J. Chevalet, A. Barhoun, F. Lantelme, Electrochemical oxidation of nickel in alkaline solutions: a voltammetric study and modeling. J. Electroanal. Chem. 442(1-2), 113–123 (1998)
S.M. Abd El Haleem, B.G. Ateya, Cyclic voltammetry of copper in sodium hydroxide solutions. J. Electroanal. Chem. 117, 3019 (1981)
Acknowledgments
The authors wish to thank Dra. Paulina Lloret and BSc. Lionel Veiga from nanomaterials group (INTI-Procesos Superficiales) for SEM images, Laboratorio de Especies Cristalinas—DRX (INTI-Química), and Eng. Shaun T. Mc Mahon (INTI-Mecánica) for language revision.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abuin, G., Coppola, R. & Diaz, L. Ni-Mo Alloy Electrodeposited over Ni Substrate for HER on Water Electrolysis. Electrocatalysis 10, 17–28 (2019). https://doi.org/10.1007/s12678-018-0490-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-018-0490-2