Abstract
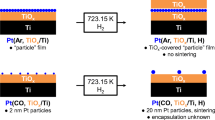
Very thin Pt layers on inexpensive substrates are promising oxygen reduction reaction (ORR) catalysts for polymer electrolyte fuel cells (PEFCs). TiOx is considered a suitable substrate but shows problems with conductivity, thus masking chemical effects by semiconductor effects (mismatch in energy states hindering electron transport). The native oxide on metallic Ti (TiOx/Ti) has been used as a novel and promising model substrate for ORR studies eliminating semiconductor effects. A high-coverage “particle” layer with high specific ORR activity was formed via electrodeposition from Ar-saturated solution. While high specific activities could be demonstrated, the concept could not be enhanced to high mass activities by limiting the Pt deposition amount. The approach to quench Pt deposition by introducing CO failed due to its adsorption to the TiOx/Ti substrate before metal deposition and thus the prevention of layer formation. A similar approach for the Pt/Au codeposition was also unsuccessful manifesting the TiOx/Ti-CO incompatibility even further.

CO, blessing, and curse: Pt deposition from Ar-saturated solution leads to a “film”-like deposit with high specific ORR activity. In contrast, the corresponding CO-saturated solution leads to deposition termination but a smooth monolayer is not formed due to interaction of CO with the TiOx/Ti substrate and, consequently, very low ORR activity is obtained.









Similar content being viewed by others
References
M. Shao, Q. Chang, J.-P. Dodelet, R. Chenitz, Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 116(6), 3594–3657 (2016)
P.C.K. Vesborg, T.F. Jaramillo, Addressing the terawatt challenge: scalability in the supply of chemical elements for renewable energy. RSC Adv. 2(21), 7933 (2012)
M.K. Debe, Electrocatalyst approaches and challenges for automotive fuel cells. Nature 486(7401), 43–51 (2012)
D.F. van der Vliet, C. Wang, D. Tripkovic, D. Strmcnik, X.F. Zhang, M.K. Debe, R.T. Atanasoski, N.M. Markovic, V.R. Stamenkovic, Mesostructured thin films as electrocatalysts with tunable composition and surface morphology. Nat. Mater. 11(12), 1051–1058 (2012)
M. Nesselberger, M. Roefzaad, R. Fayçal Hamou, P. Ulrich Biedermann, F.F. Schweinberger, S. Kunz, K. Schloegl, G.K.H. Wiberg, S. Ashton, U. Heiz, K.J.J. Mayrhofer, M. Arenz, The effect of particle proximity on the oxygen reduction rate of size-selected platinum clusters. Nat. Mater. 12(10), 919–924 (2013)
I.Harkness, J.Sharman, M.Bosund, T.Geppert, H.El-Sayed, H. A.Gasteiger, G.Ercolano, S.Cavaliere, D.Jones, J. Roziere, Demonstration of Pt-catalysed non-carbon support with higher mass activity than conventional Pt/C nanoparticles and in excess of 0.15 A/Mg Pt, 2014
I.Harkness, J.Sharman, Fibrous Pt catalysts created with ALD-deposited Pt on oxide, carbide or nitride surface tie layers where the Pt deposits extend over the surface in large contiguous islands or as continuous film, 2014
S.M. Alia, B.A. Larsen, S. Pylypenko, D.A. Cullen, D.R. Diercks, K.C. Neyerlin, S.S. Kocha, B.S. Pivovar, Platinum-Coated Nickel Nanowires as Oxygen-Reducing Electrocatalysts. ACS Catal. 4, 1114–1119 (2014)
S. Proch, K. Kodama, S. Yoshino, N. Takahashi, N. Kato, Y. Morimoto, CO-Terminated Platinum Electrodeposition on Nb-Doped Bulk Rutile TiO2. Electrocatalysis 7(5), 362–375 (2016)
G. A.Somorjai, Y.Li, Introduction to Surface Chemistry and Catalysis, Second Edition, (John Wiley & Sons, Inc., 2010)
S.R. Brankovic, J.X. Wang, R.R. Adžić, Metal monolayer deposition by replacement of metal adlayers on electrode surfaces. Surf. Sci. 474(1-3), L173–L179 (2001)
K. Sasaki, Y. Mo, J.X. Wang, M. Balasubramanian, F. Uribe, J. McBreen, R.R. Adzic, Pt submonolayers on metal nanoparticles—novel electrocatalysts for H2 oxidation and O2 reduction. Electrochim. Acta 48(25-26), 3841–3849 (2003)
Y. Liu, D. Gokcen, U. Bertocci, T.P. Moffat, Self-terminating growth of platinum films by electrochemical deposition. Science 338(6112), 1327–1330 (2012)
Y.-J. Deng, V. Tripkovic, J. Rossmeisl, M. Arenz, Oxygen reduction reaction on Pt overlayers deposited onto a gold film: ligand, strain, and ensemble effect. ACS Catal. 6(2), 671–676 (2016)
S. Brimaud, R.J. Behm, Electrodeposition of a Pt monolayer film: using kinetic limitations for atomic layer epitaxy. J. Am. Chem. Soc. 135(32), 11716–11719 (2013)
J. Speder, L. Altmann, M. Baumer, J.J.K. Kirkensgaard, K. Mortensen, M. Arenz, The particle proximity effect: from model to high surface area fuel cell catalysts. RSC Adv. 4(29), 14971 (2014)
J. Speder, L. Altmann, M. Roefzaad, M. Baumer, J.J.K. Kirkensgaard, K. Mortensen, M. Arenz, Pt based PEMFC catalysts prepared from colloidal particle suspensions—a toolbox for model studies. Phys. Chem. Chem. Phys. 15(10), 3602–3608 (2013)
S. Proch, K. Kodama, M. Inaba, K. Oishi, N. Takahashi, Y. Morimoto, The “particle proximity effect” in three dimensions: a case study on Vulcan XC 72R. Electrocatalysis 7(3), 249–261 (2016)
J. Speder, I. Spanos, A. Zana, J.J.K. Kirkensgaard, K. Mortensen, L. Altmann, M. Bäumer, M. Arenz, From single crystal model catalysts to systematic studies of supported nanoparticles. Surf. Sci. 631, 278–284 (2015)
R. Borup, J. Meyers, B. Pivovar, Y.S. Kim, R. Mukundan, N. Garland, D. Myers, M. Wilson, F. Garzon, D. Wood, P. Zelenay, K. More, K. Stroh, T. Zawodzinski, J. Boncella, J.E. McGrath, M. Inaba, K. Miyatake, M. Hori, K. Ota, Z. Ogumi, S. Miyata, A. Nishikata, Z. Siroma, Y. Uchimoto, K. Yasuda, K.-i. Kimijima, N. Iwashita, Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 107(10), 3904–3951 (2007)
H.A. Gasteiger, S.S. Kocha, B. Sompalli, F.T. Wagner, Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal., B 56(1-2), 9–35 (2005)
J. Parrondo, T. Han, E. Niangar, C. Wang, N. Dale, K. Adjemian, V. Ramani, Platinum supported on titanium-ruthenium oxide is a remarkably stable electrocatayst for hydrogen fuel cell vehicles. Proc. Natl. Acad. Sci. U. S. A. 111(1), 45–50 (2014)
A.Michaelis, in Advances in Electrochemical Science and Engineering, ed. By R. C.Alkire, D. M.Kolb, J.Lipkowski,P. N.Ross (WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2008), p. 1
N.P. Subramanian, S.P. Kumaraguru, H. Colon-Mercado, H. Kim, B.N. Popov, T. Black, D.A. Chen, Studies on Co-based catalysts supported on modified carbon substrates for PEMFC cathodes. J. Power Sources 157(1), 56–63 (2006)
K. Lee, A. Mazare, P. Schmuki, One-dimensional titanium dioxide nanomaterials: nanotubes. Chem. Rev. 114(19), 9385–9454 (2014)
M. Nakada, A. Ishihara, S. Mitsushima, N. Kamiya, K.-i. Ota, Effect of tin oxides on oxide formation and reduction of platinum particles. Electrochem. Solid-State Lett. 10(1), F1 (2007)
B.E. Hayden, Acc. Chem. Res. 46 (1858, 2013)
B.E. Hayden, D. Pletcher, J.-P. Suchsland, L.J. Williams, The influence of support and particle size on the platinum catalysed oxygen reduction reaction. Phys. Chem. Chem. Phys. 11(40), 9141–9148 (2009)
D. Schäfer, C. Mardare, A. Savan, M.D. Sanchez, B. Mei, W. Xia, M. Muhler, A. Ludwig, W. Schuhmann, High-throughput characterization of Pt supported on thin film oxide material libraries applied in the oxygen reduction reaction. Anal. Chem. 83(6), 1916–1923 (2011)
C.A. Koval, J.N. Howard, Electron transfer at semiconductor electrode-liquid electrolyte interfaces. Chem. Rev. 92(3), 411–433 (1992)
C. Kim, S. Kim, J. Choi, J. Lee, J.S. Kang, Y.-E. Sung, J. Lee, W. Choi, J. Yoon, Blue TiO2 nanotube array as an oxidant generating novel anode material fabricated by simple cathodic polarization. Electrochim. Acta 141, 113–119 (2014)
R.T. Tung, Mater. Sci.Eng., R 35, 1 (2001)
H. Gerischer, The impact of semiconductors on the concepts of electrochemistry. Electrochim. Acta 35(11-12), 1677–1699 (1990)
H.Gerischer, in Top. Appl. Phys., ed. By B. O.Seraphin (Springer, Berlin-Heidelberg, 1979), p. 115
R. Hahn, F. Schmidt-Stein, J. Salonen, S. Thiemann, Y. Song, J. Kunze, V.-P. Lehto, P. Schmuki, Semimetallic TiO2 nanotubes. Angew. Chem. Int. Ed. 48(39), 7236–7239 (2009)
S. Proch, S. Yoshino, N. Kato, N. Takahashi, Y. Morimoto, Titania nanotube arrays (TNAs) as support for oxygen reduction reaction (ORR) platinum thin film catalysts. Electrocatalysis 7(6), 451–465 (2016)
S. Proch, S. Yoshino, I. Gunjishima, S. Kosaka, N. Takahashi, N. Kato, K. Kodama, Y. Morimoto, Acetylene-treated titania nanotube arrays (TNAs) as support for oxygen reduction reaction (ORR) platinum thin film catalysts. Electrocatalysis 8(4), 351–365 (2017)
M.S. Chen, D.W. Goodman, The structure of catalytically active gold on titania. Science 306(5694), 252–255 (2004)
S. Proch, S. Yoshino, N. Takahashi, S. Kosaka, K. Kodama, Y. Morimoto, CO-terminated Pt/Au codeposition on titania nanotube arrays (TNAs). Electrocatalysis 8(5), 480–491 (2017)
D.W. Goodman, Model catalysts: from imagining to imaging a working surface. J. Catal. 216(1-2), 213–222 (2003)
J.W. Schultze, M.M. Lohrengel, Stability, reactivity and breakdown of passive films. Problems of recent and future research. Electrochim. Acta 45(15-16), 2499–2513 (2000)
M.Pourbaix, Atlas of Electrochemical Equilibria in Aqueous Solutions,2 (National Association of Corrosion Engineers, 1974)
P. Schmuki, From Bacon to barriers: a review on the passivity of metals and alloys. J. Solid State Electrochem. 6(3), 145–164 (2002)
A.K. Sharma, Anodizing titanium for space applications. Thin Solid Films 208(1), 48–54 (1992)
J. Biedrzycki, S. Livraghi, E. Giamello, S. Agnoli, G. Granozzi, Fluorine- and niobium-doped TiO2: chemical and spectroscopic properties of polycrystalline n-type-doped anatase. J. Phys. Chem. C 118(16), 8462–8473 (2014)
J. F.Moulder, W. F.Stickle, P. E.Sobol, K. D. Bomben, in Handbook of X-Ray Photoelectron Spectroscopy, (Physical Electronics, Inc., 1995)
D.S. Ghosh, Basics of ultrathin metal films and their use as transparent electrodes (Springer International Publishing, Heidelberg, 2013)
Z.H. Lu, J.P. McCaffrey, B. Brar, G.D. Wilk, R.M. Wallace, L.C. Feldman, S.P. Tay, SiO2 film thickness metrology by x-ray photoelectron spectroscopy. Appl. Phys. Lett. 71(19), 2764–2766 (1997)
S. Muhammad Rizwan, H. Seppo, T. Jari, IOP Conf Ser Mater Sci Eng 60, 012008 (2014)
Y. Garsany, O.A. Baturina, K.E. Swider-Lyons, S.S. Kocha, Experimental methods for quantifying the activity of platinum electrocatalysts for the oxygen reduction reaction. Anal. Chem. 82(15), 6321–6328 (2010)
D. Sazou, K. Saltidou, M. Pagitsas, Understanding the effect of bromides on the stability of titanium oxide films based on a point defect model. Electrochim. Acta 76, 48–61 (2012)
C. Rüdiger, F. Maglia, S. Leonardi, M. Sachsenhauser, I.D. Sharp, O. Paschos, J. Kunze, Surface analytical study of carbothermally reduced titania films for electrocatalysis application. Electrochim. Acta 71, 1–9 (2012)
A. Linsebigler, G. Lu, J.T. Yates, J. Chem. Phys. 103(21), 9438–9443 (1995)
W. Göpel, G. Rocker, R. Feierabend, Intrinsic defects of TiO2(110): interaction with chemisorbed O2, H2, CO, and CO2. Phys. Rev. B 28(6), 3427–3438 (1983)
G.B. Raupp, J.A. Dumesic, Adsorption of carbon monoxide, carbon dioxide, hydrogen, and water on titania surfaces with different oxidation states. J. Phys. Chem. 89(24), 5240–5246 (1985)
A. J.Bard, L. R.Faulkner, Electrochemical Methods—Fundamentals and Applications, Second Edition, (John Wiley & Sons, Inc., New York, 2001)
X.-Q. Gong, A. Selloni, O. Dulub, P. Jacobson, U. Diebold, Small Au and Pt clusters at the anatase TiO2(101) surface: behavior at terraces, steps, and surface oxygen vacancies. J. Am. Chem. Soc. 130(1), 370–381 (2008)
S. Trasatti, O.A. Petrii, Pure Appl. Chem. 63, 711 (1991)
J.C. Calabrese, L.F. Dahl, P. Chini, G. Longoni, S. Martinengo, Synthesis and structural characterization of platinum carbonyl cluster dianions bis, tris, tetrakis, or pentakis (tri-.mu.2-carbonyl-tricarbonyltriplatinum)(2-). New series of inorganic oligomers. J. Am. Chem. Soc. 96(8), 2614–2616 (1974)
G. Longoni, P. Chini, Synthesis and chemical characterization of platinum carbonyl dianions [Pt3(CO)6]n2- (n = .apprx.10,6,5,4,3,2,1). A new series of inorganic oligomers. J. Am. Chem. Soc. 98(23), 7225–7231 (1976)
H. Inada, D. Su, R.F. Egerton, M. Konno, L. Wu, J. Ciston, J. Wall, Y. Zhu, Atomic imaging using secondary electrons in a scanning transmission electron microscope: experimental observations and possible mechanisms. Ultramicroscopy 111(7), 865–876 (2011)
G.N. Derry, P.N. Ross, High coverage states of oxygen adsorbed on Pt(100) and Pt(111) surfaces. Surf. Sci. 140(1), 165–180 (1984)
L. Calvillo, D. Fittipaldi, C. Rüdiger, S. Agnoli, M. Favaro, C. Valero-Vidal, C. Di Valentin, A. Vittadini, N. Bozzolo, S. Jacomet, L. Gregoratti, J. Kunze-Liebhäuser, G. Pacchioni, G. Granozzi, Carbothermal transformation of TiO2 into TiOxCy in UHV: tracking intrinsic chemical stabilities. J. Phys. Chem. C 118(39), 22601–22610 (2014)
T.L. Barr, S. Seal, J. Vac, Sci. Technol., A 13, 1239 (1995)
E. Wahlström, N. Lopez, R. Schaub, P. Thostrup, A. Rønnau, C. Africh, E. Lægsgaard, J.K. Nørskov, F. Besenbacher, Bonding of gold nanoclusters to oxygen vacancies on rutile TiO2(110). Phys. Rev. Lett. 90(2), 026101 (2003)
B.K. Min, W.T. Wallace, D.W. Goodman, Synthesis of a sinter-resistant, mixed-oxide support for Au nanoclusters†. J. Phys. Chem. B 108(38), 14609–14615 (2004)
L.D. Burke, Platin. Met. Rev. 38, 166 (1994)
B.B. Blizanac, C.A. Lucas, M.E. Gallagher, M. Arenz, P.N. Ross, N.M. Marković, Anion adsorption, CO oxidation, and oxygen reduction reaction on a Au(100) surface: the pH effect. J. Phys. Chem. B 108(2), 625–634 (2004)
W.S. Baker, J.J. Pietron, M.E. Teliska, P.J. Bouwman, D.E. Ramaker, K.E. Swider-Lyons, Enhanced oxygen reduction activity in acid by tin-oxide supported Au nanoparticle catalysts. J. Electrochem. Soc. 153(9), A1702 (2006)
B.E. Hayden, D. Pletcher, J.-P. Suchsland, L.J. Williams, The influence of Pt particle size on the surface oxidation of titania supported platinum. Phys. Chem. Chem. Phys. 11(10), 1564–1570 (2009)
L. Timperman, A. Lewera, W. Vogel, N. Alonso-Vante, Nanostructured platinum becomes alloyed at oxide-composite substrate. Electrochem. Commun. 12(12), 1772–1775 (2010)
W. Vogel, L. Timperman, N. Alonso-Vante, Probing metal substrate interaction of Pt nanoparticles: structural XRD analysis and oxygen reduction reaction. Appl. Catal., A 377(1-2), 167–173 (2010)
L. Timperman, Y.J. Feng, W. Vogel, N. Alonso-Vante, Substrate effect on oxygen reduction electrocatalysis. Electrochim. Acta 55(26), 7558–7563 (2010)
K.J.J. Mayrhofer, D. Strmcnik, B.B. Blizanac, V. Stamenkovic, M. Arenz, N.M. Markovic, Measurement of oxygen reduction activities via the rotating disc electrode method: from Pt model surfaces to carbon-supported high surface area catalysts. Electrochim. Acta 53(7), 3181–3188 (2008)
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
ESM 1
(DOCX 1002kb)
Rights and permissions
About this article
Cite this article
Proch, S., Yoshino, S., Takahashi, N. et al. The Native Oxide on Titanium Metal as a Conductive Model Substrate for Oxygen Reduction Reaction Studies. Electrocatalysis 9, 608–622 (2018). https://doi.org/10.1007/s12678-018-0465-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-018-0465-3