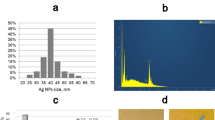
Abstract
Purulent wounds are associated with microbial persistence that alters healing and can lead to septic complications. Infected wound management includes use of antibacterial agents for providing fast and complete bacterial elimination with no adverse effects on tissue repair. The performance of chlorhexidine (Ch) irrigation as the main strategy for purulent wound treatment is still under debates and stimulates further development of antimicrobial strategies. In this study, the effects of silver nanoparticles (AgNP) on infected wound microbiology and healing were assessed in vitro and in vivo. AgNPs provided high antibacterial activity at the minimal inhibitory concentration (MIC) of 1.25 μg/mL for E. coli and P. aeruginosa, and 2.5 μg/mL for S. aureus. At the same time assessment of AgNPs, cytotoxicity in fibroblast culture demonstrated high cytocompatibility in concentrations 5 times higher than MIC. These data agreed with in vivo studies with purulent wound modeling followed by use of three treatment options: Ch-, AgNP, and Ch+AgNP wound dressings. It was shown that AgNP treatment effectively decreased the microbial contamination but had only slight effect on wound healing parameters. In contrast, Ch+AgNP combination was associated with significant acceleration of wound clearance and closure related with early M2 macrophage polarization and an enhanced healing process. In conclusion, adding of AgNPs to chlorhexidine treatment improves infected wound healing through acceleration of bacteria elimination and M2 macrophage polarization. Synergic effects of AgNPs and chlorhexidine could be a promising option for optimizing infected wound management.
Graphical abstract












Similar content being viewed by others
References
Young, P. Y., & Khadaroo, R. G. (2014). Surgical site infections. Surg Clin N Am, 94, 1245–1264.
Fry, D. E. (2011). Fifty ways to cause surgical site infections. Surg Infect (Larchmt), 12(6), 497–500.
Stone, P. W., Braccia, D., & Larson, E. (2011). Systematic review of economic analyses of health care–associated infections. Am J Infect Control., 33(9), 501–509.
Leaper, D., & Ousey, K. (2015). Evidence update on prevention of surgical site infection. Curr Opin Infect Dis, 28(2), 158 63.
Ling, et al. (2019). APSIC guidelines for the prevention of surgical site infections. Antimicrobial Resistance and Infection Control. https://doi.org/10.1186/s13756-019-0638-8.
Di Piro, J. T., Martindale, R. G., Bakst, A., et al. (1998). Infection in surgical patients: effects on mortality, hospitalization. and postdischarge care. Am J Health Syst Pharm, 55(8), 777–781.
Turner, N. A., Sharma-Kuinkel, B. K., Maskarinec, S. A., Eichenberger, E. M., Shah, P. P., Carugati, M., Holland, T. L., & Fowler Jr., V. G. (2019). Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev. Microbiol, 17(4), 203–218.
Sievert, D. M., Ricks, P., Edwards, J. R., et al. (2013). Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol, 34(1), 1–14.
Anderson, D. J., Sexton, D. J., Kanafani, Z. A., et al. (2007). Severe surgical site infection in community hospitals: epidemiology, key procedures, and the changing prevalence of methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol, 28(9), 1047–1053.
Mangram, A. J., Horan, T. C., Pearson, M. L., et al. (1999). Guideline for prevention of surgical site infection. Am J Infect Control, 27(2), 97–134.
Lee, I., Agarwal, R. K., Lee, B. Y., et al. (2010). Systematic review and cost analysis comparing use of chlorhexidine with use of iodine for preoperative skin antisepsis to prevent surgical site infection. Infect Control Hosp Epidemiol, 31(12), 1219–1229.
Swenson, B. R., Hedrick, T. L., Metzger, R., et al. (2009). Effects of preoperative skin preparation on postoperative wound infection rates: a prospective study of 3 skin preparation protocols. Infect Control Hosp Epidemiol, 30(10), 964–971.
Hakkarainen, T. W., Dellinger, E. P., Evans, H. L., et al. (2014). Comparative effectiveness of skin antiseptic agents in reducing surgical site infections: a report from the Washington State Surgical Care and Outcomes Assessment Program. J Am Coll Surg, 218(3), 336–344.
Roy, S., Shankar, S., & Rhim, J. W. (2019). Melanin-mediated synthesis of silver nanoparticle and its use for the preparation of carrageenan-based antibacterial films. Food Hydrocoll, 88, 237–246.
Liu, X., Gao, P., Du, J., Zhao, X., & Wong, K. K. Y. (2017). Long-term anti-inflammatory efficacy in intestinal anastomosis in mice using silver nanoparticle-coated suture. J Pediatr Surg, 52, 2083–2087.
Choudhury, H., et al. (2020). Silver nanoparticles: advanced and promising technology in diabetic wound therapy. Materials Science & Engineering C, 112, 110925.
Das, S., & Baker, A. B. (2016). Biomaterials and nanotherapeutics for enhancing skin wound healing. Front Bioeng Biotechnol, 4, 82.
Hamdan, S., Pastar, I., Drakulich, S., Dikici, E., Tomic-Canic, M., Deo, S., & Daunert, S. (2017). Nanotechnology-driven therapeutic interventions in wound healing: potential uses and applications. ACS Cent Sci, 3, 163–175.
Burdușel, A. C., Gherasim, O., Grumezescu, A. M., Mogoantă, L., Ficai, A., & Andronescu, E. (2018). Biomedical applications of silver nanoparticles: an up-to-date overview. Nanomaterials, 8, E681.
Rajkumar, R. J., Nadar, M. M., & Mosae Selvakumar, P. (2017). Nanotechnology in wound healing-a review. Glob J Nanomed, 3, 555605.
Zarrintaj, P., Moghaddam, A. S., Manouchehri, S., Atoufi, Z., Amiri, A., Amirkhani, M. A., Nilforoushzadeh, M. A., Saeb, M. R., Hamblin, M. R., & Mozafari, M. (2017). Can regenerative medicine and nanotechnology combine to heal wounds? The search for the ideal wound dressing. Nanomedicine, 12, 2403–2422.
Hajimiri, M., Shahverdi, S., Esfandiari, M. A., Larijani, B., Atyabi, F., Rajabiani, A., Dehpour, A. R., Amini, M., & Dinarvand, R. (2016). Preparation of hydrogel embedded polymer-growth factor conjugated nanoparticles as a diabetic wound dressing. Drug Dev Ind Pharm, 42, 707–719.
Reinke, J. M., & Sorg, H. (2012). Wound Repair and Regeneration. Eur Surg, 49, 35–43.
Han, G., & Ceilley, R. (2017). Chronic wound healing: a review of current management and treatments. Adv Ther, 34(3), 599–610.
Eming, S. A., Krieg, T., & Davidson, J. M. (2007). Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol, 127, 514–525.
Werner, S., & Grose, R. (2003). Regulation of wound healing by growth factors and cytokines. Physiol Rev., 83, 835–870.
Snyder, R. J., Lantis, J., Kirsner, R. S., Shah, V., Molyneaux, M., & Carter, M. J. (2016). Macrophages: a review of their role in wound healing and their therapeutic use. Wound Repair Regen, 24(4), 613–629.
Wang, Y., Smith, W., Hao, D., He, B., & Kong, L. (2019). M1 and M2 macrophage polarization and potentially therapeutic naturally occurring compounds. Int Immunopharmacol, 70, 459–466.
Li, N., Liu, Y., & Cai, J. (2019). LncRNA MIR155HG regulates M1/M2 macrophage polarization in chronic obstructive pulmonary disease. Biomed Pharmacother, 117, 109015.
Varela, P., Marlinghaus, L., Sartori, S., Viebahn, R., Salber, J., & Ciardelli, G. (2020). Response of human macrophages to clinically applied wound dressings loaded with silver. Front Bioeng Biotechnol, 8, 124.
Yamamichi, K., Fukuda, T., Sanui, T., Toyoda, K., Tanaka, U., Nakao, Y., Yotsumoto, K., Yamato, H., Taketomi, T., Uchiumi, T., & Nishimura, F. (2017). Amelogenin induces M2 macrophage polarisation via PGE2/cAMP signalling pathway. Arch Oral Biol, 83, 241–251.
Funding
This research was funded by EU-H2020-MSCA-RISE, grant no. 777926 NanoSurf. The authors acknowledge the grants from the Ministry of Education and Science of Ukraine (0119U100823 and 0118U003577) for support in cell culture and bacteriological research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Research Involving Humans and Animal
Seventy-two laboratory rats from Vivarium of Sumy State University were used in the experiment. Keeping, feeding, handling of animals, and all experiments were carried out in accordance with the Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. All the procedures performed in the study were approved by the Commission on Bioethics Compliance in Experimental and Clinical Research.
Informed Consent
The research did not involve human subjects or personal information. No any informed consent required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Myronov, P., Sulaieva, O., Korniienko, V. et al. Combination of Chlorhexidine and Silver Nanoparticles: an Efficient Wound Infection and Healing Control System. BioNanoSci. 11, 256–268 (2021). https://doi.org/10.1007/s12668-021-00834-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-021-00834-5