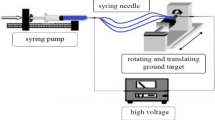
Abstract
Albendazole (ABZ) is a broad-spectrum, synthetic benzimidazole anthelmintic approved by the US FDA in 1996. However, it exhibits low oral bioavailability owing to its high lipophilicity and poor water solubility. Moreover, if such a drug molecule is projected for the therapy of a particular group of the population, for example, pediatric or geriatric, the formulation challenge is even greater with the need to manufacture a dosage form that is suitable for particular patients. Hence, the aim of the present study was to investigate electrospun polyvinyl alcohol (PVA) nanofiber films as a new delivery system adopted for the oromucosal administration of ABZ. The prepared nanofiber films were evaluated regarding their mechanical and physicochemical properties, in vitro drug release, and ex vivo permeation. Scanning electron microscopy was used to prove the formation of uniform, non-woven, smooth nanofibers. ABZ-loaded PVA nanofiber films showed ~ 5-fold improvement in the dissolution rate of ABZ as compared to ABZ alone. Additionally, an ex vivo permeation study in a goat buccal mucosa displayed 3.2-fold improvement in the permeation of ABZ from PVA nanofiber films. The prepared nanofiber films were found to be stable over the period of 3 months, confirmed from accelerated stability studies. Accordingly, electrospinning was shown to be a capable nanotechnology-based strategy for the formulation of poorly water-soluble molecules so as to improve their primary and secondary biopharmaceutical properties. Thus, oromucosal administration of drug-loaded nanofiber films could be a promising approach for pediatric or geriatric patients.









Similar content being viewed by others
References
Kamble, R., Bothiraja, C., Mehta, P., & Varghese, V. (2017). Synthesis, solid state characterization and antifungal activity of ketoconazole cocrystals. Journal of Pharmaceutical Investigation. https://doi.org/10.1007/s40005-017-0346-4.
Kamble, R., Sharma, S., & Mehta, P. (2017). Norfloxacin mixed solvency based solid dispersions: an in-vitro and in-vivo investigation. Journal of Taibah University for Science, 11, 512–522.
Dhapte, V., & Mehta, P. (2015). Advances in hydrotropic solutions: an updated review. St. Petersburg Polytechnical University Journal: Physics and Mathematics, 1, 424–435.
Kamble, R., Mehta, P., & Kumar, A. (2016). Efavirenz self-nano-emulsifying drug delivery system: in vitro and in vivo evaluation. AAPS PharmSciTech, 17, 1240–1247.
Hearnden, V., Sankar, V., Hull, K., Juras, D., Greenberg, M., Kerr, A., Lockhart, P., Patton, L., Porter, S., & Thornhill, M. (2012). New developments and opportunities in oral mucosal drug delivery for local and systemic disease. Advanced Drug Delivery Reviews, 64, 16–28.
Gegel, N., Abramov, A., Shipovskaya, A., & Zudina, I. (2016). A biocidal nanocoating based on the macromolecular chitosan acetate-sodium albuminate-silver nanoparticles system. BioNanoSci. https://doi.org/10.1007/s12668-016-0210-4.
Parejiya, P., Patel, R., Mehta, D., Shelat, P., & Barot, B. (2013). Quick dissolving films of nebivolol hydrochloride: formulation and optimization by a simplex lattice design. Journal of Pharmaceutical Investigation, 43, 343–351.
Vijayanand, P., Patil, J., & Reddy, M. (2015). Formulation and comparative pharmacokinetic evaluation of orodispersible tablets and films of nebivolol hydrochloride. Journal of Pharmaceutical Investigation, 45, 237–247.
Colombo, P., Cagnani, S., Sonvico, P., & Santi, P. (2007). Biological in vitro models for absorption by non oral routes. Comprehensive Medicinal Chemistry II, 5, 279–299.
Pawar, A., Rajalakshmi, S., Mehta, P., Shaikh, K., & Bothiraja, C. (2016). Strategies for formulation development of andrographolide. RSC Advances, 6, 69282–69300.
Bhardwaj, N., & Kundu, S. (2010). Electrospinning: a fascinating fiber fabrication technique. Biotechnology Advances, 28, 325–347.
Bakhtiari, M., Salehi, R., Akbarzadeh, A., & Davaran, S. (2017). Development of novel doxorubicin loaded biodegradable polymeric nanofibers as the anticancer drug delivery systems. BioNanoSci. https://doi.org/10.1007/s12668-017-0421-3.
Reda, R., Wen, M., & El-Kamel, A. (2017). Ketoprofen-loaded Eudragit electrospun nanofibers for the treatment of oral mucositis. International Journal of Nanomedicine, 12, 2335–2351.
Kamble, R., Gaikwad, S., Maske, A., & Patil, S. (2016). Fabrication of electrospun nanofibers of BCS II drug for enhanced dissolution and permeation across skin. Journal of Advanced Research, 7, 483–489.
Bhandari, J., Mishra, H., Mishra, P., Wimmer, R., Ahmad, F., & Talegaonkar, S. (2017). Cellulose nanofiber aerogel as a promising biomaterial for customized oral drug delivery. International Journal of Nanomedicine, 12, 2021–2031.
Kadajji, V., & Betageri, G. (2011). Water soluble polymers for pharmaceutical applications. Polymers, 3, 1972–2009.
Dayan, A. (2003). Albendazole, mebendazole and praziquantel. Review of non-clinical toxicity and pharmacokinetics. Acta Tropica, 86, 141–159.
Mansuri, S., Kesharwani, P., Tekade, R., & Jain, N. (2016). Lyophilized mucoadhesive-dendrimer enclosed matrix tablet for extended oral delivery of albendazole. European Journal of Pharmaceutics and Biopharmaceutics, 102, 202–213.
Ahmadnia, S., Moazeni, M., Mohammadi-Samani, S., & Oryan, A. (2013). In vivo evaluation of the efficacy of albendazole sulfoxide and albendazole sulfoxide loaded solid lipid nanoparticles against hydatid cyst. Experimental Parasitology, 135, 314–319.
Casulli, A., Morales, M., Gallinella, B., Turchetto, L., & Pozio, E. (2006). 2-Hydroxypropyl-b-cyclodextrin improves the effectiveness of albendazole against encapsulated larvae of Trichinella spiralis in a murine model. The Journal of Antimicrobial Chemotherapy, 58, 886–890.
Ibrahim, M., Shazly, G., & El-Badry, M. (2014). Albendazole microparticles prepared by spray drying technique: improvement of drug dissolution. Tropical Journal of Pharmaceutical Research, 13, 1963–1970.
Meena, A., Sharma, K., Kandaswamy, M., Rajagopal, S., & Mullangi, R. (2012). Formulation development of an albendazole self-emulsifying drug delivery system (SEDDS) with enhanced systemic exposure. Acta Pharmaceutica, 62, 563–580.
Shinde, R., Bharkad, G., & Devarajan, P. (2015). Intranasal microemulsion for targeted nose to brain delivery in neurocysticercosis: role of docosahexaenoic acid. European Journal of Pharmaceutics and Biopharmaceutics, 96, 363–379.
Kraisit, P., Limmatvapirat, S., Luangtana-Anan, M., & Sriamornsak, P. (2018). Buccal administration of mucoadhesive blend films saturated with propranolol loaded nanoparticles. Asian Journal of Pharmaceutical Science, 13, 34–43.
Liew, K., Tan, Y., & Peh, K. (2011). Characterization of oral disintegrating film containing donepezil for alzheimer disease. AAPS PharmSciTech, 13, 134–142.
Aydogdu, M., Oprea, A., Trusca, R., Surdu, A., Ficai, A., Holban, A., Iordache, F., Paduraru, A., Filip, D., Altun, E., Ekren, N., Oktar, F., & Gunduz, O. (2017). Production and characterization of antimicrobial electrospun nanofibers containing polyurethane, zirconium oxide and zeolite. BioNanoSci. https://doi.org/10.1007/s12668-017-0443-x.
Goreninskii, S., Stankevich, K., Bolbasov, E., Danilenko, N., Filimonov, V., & Tverdokhlebov, S. (2017). Surface modification of PLLA electrospun nanofiber materials for biomedical applications. BioNanoSci. https://doi.org/10.1007/s12668-017-0422-2.
Bothiraja, C., Shinde, M., Rajalakshmi, S., & Pawar, A. (2009). Evaluation of molecular pharmaceutical and in-vivo properties of spray-dried isolated andrographolide-PVP. The Journal of Pharmacy and Pharmacology, 61, 1465–1472.
Hanif, M., & Zaman, M. (2017). Thiolation of arabinoxylan and its application in the fabrication of controlled release mucoadhesive oral films. Daru, 25, 1–13.
Bothiraja, C., Pawar, A., Dama, G., Joshi, P., & Shaikh, K. (2012). Novel solvent free gelucire extract of Plumbago zeylanica using non-everted rat intestinal sac method for improved therapeutic efficacy of plumbagin. Journal of Pharmacology and Toxicology, 66, 35–42.
World Health Organization. (2009). Annex 2, WHO technical report series, no. 953, stability testing of active pharmaceutical ingredients and finished pharmaceutical products. Section 2.2.6, 101–103.
Poralan, G., Gambe, J., Alcantara, E., & Vequizo, R. (2015). X-ray diffraction and infrared spectroscopy analyses on the crystallinity of engineered biological hydroxyapatite for medical application. IOP Conference Series: Materials Science and Engineering, 79, 1–7.
Kamble, R., Palve, P., & Mehta, P. (2014). Preparation and evaluation of amorphous olmesartan medoxomil with porous silica microparticles using spray-drying technique. Journal of Advanced Pharmacy Education and Research, 4, 65–71.
Mehta, P., & Dhapte, V. (2014). Propulsive PAT paradigm: optimization of freeze drying process. International Journal of Pharmaceutical Sciences Review and Research, 28, 240–246.
Potrc, T., Baumgartner, S., Roskar, R., Planinsek, O., Lavric, Z., Kristl, J., & Kocbek, P. (2015). Electrospun polycaprolactone nanofibers as a potential oromucosal delivery system for poorly water-soluble drugs. European Journal of Pharmaceutical Sciences, 75, 101–113.
Vuddanda, P., Mathew, A., & Velaga, S. (2016). Electrospun nanofiber mats for ultrafast release of ondansetron. Reactive and Functional Polymers, 99, 65–72.
Kwak, H., Woo, H., Kim, I., & Lee, K. (2017). Fish gelatin nanofibers prevent drug crystallization and enable ultrafast delivery. RSC Advances, 7, 40411–40417.
Mehta, P., & Pawar, V. (2018). Electrospun nanofiber scaffolds: technology and applications. Applications of nanocomposite materials in drug delivery (pp. 509–573). Sawston: Woodhead Publishing Series in Biomaterials.
Dott, C., Tyagi, C., Tomar, L., Choonara, Y., Kumar, P., du Toit, L., & Pillay, V. (2013). A mucoadhesive electrospun nanofibrous matrix for rapid oramucosal drug delivery. Journal of Nanomaterials, 2013, 1–19.
Acknowledgements
The authors would like to acknowledge the support from Bharati Vidyapeeth (Deemed to be) University, Poona College of Pharmacy, Pune, Maharashtra, India.
Funding
There are no funding agencies to report for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Statements
The present work does not involve any animal or human volunteer.
Conflict of Interest
The authors declare that they have no conflict of interest.
Research Involving Humans and Animals Statement
None.
Informed consent
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kamble, R.N., Mehtre, R.V., Mehta, P.P. et al. Albendazole Electrospun Nanofiber Films: In-vitro and Ex-vivo Assessment. BioNanoSci. 9, 625–636 (2019). https://doi.org/10.1007/s12668-019-00627-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-019-00627-x