Abstract
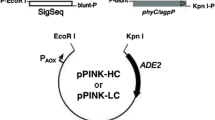
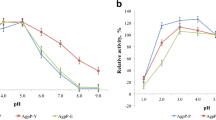
The dimorphic yeasts Yarrowia lipolytica are used as effective expression system and are characterized by a high level of production of heterologous protein. In this work, we aimed to clone and express Pantoea sp. 3.5.1 agpP phytase gene in Yarrowia lipolytica. Genetic constructs containing the native phytase gene (agpP) under the control of the strong hybrid promoter hp4d and the signal peptide of the alkaline extracellular protease XPR2 gene, as well as the optimized gene (agpP-opt) of phytase under the control of two signal peptides—bacterial and yeast were obtained. Recombinant Y. lipolytica strains with integrated bacterial phytase genes were obtained and expression of phytase was analyzed.



Similar content being viewed by others
References
Suzuki, U., Yoshimura, K., & Takaishi, M. (1907). Über ein enzym ‘Phytase’ das anhydro-oxy-methylen diphosphorsaure’ spalter. Bulletin of the College of Agriculture, Tokyo Imperial University, 7, 503–512.
Hayakawa, T., Suzuki, K., Miura, H., Ohno, T., & Igaue, I. (1990). Myo-inositol polyphosphate intermediates in the dephosphorylation of Phytic acid by acid phosphatase with phytase activity from rice bran. Agricultural and Biological Chemistry, 54(2), 279–286.
Thomas, M. P., Mills, S. J., & Potter, B. V. L. (2016). The “other” Inositols and their phosphates: synthesis, biology, and medicine (with recent advances in myoInositol chemistry). Angewandte Chemie, International Edition, 55, 1614–1650. https://doi.org/10.1002/anie.201502227.
Irvine, R. F., & Schell, M. J. (2001). Back in the water: the return of the inositol phosphates. Nature Reviews, 1, 327–338. https://doi.org/10.1038/35073015.
Haefner, S., Knietsch, A., Scholten, E., Braun, J., Lohscheidt, M., & Zelder, O. (2005). Biotechnological production and applications of phytases. Applied Microbiology and Biotechnology, 68, 588–597. https://doi.org/10.1007/s00253-005-0005-y.
Dvorakova, J., Kopecky, J., Havlicek, V., & Kren, V. (2000). Formation of myo-inositol phosphates by Aspergillus niger 3-phytase. Folia Microbiologica, 45(2), 128–132.
Siren M. (1986a). Stabilized pharmaceutical and biological material composition. Pat. SE 003165.
Siren M. (1986b). New myo-inositol triphosphoric acid isomer. Pat.SW 052950.
Suleimanova, A. D., Beinhauer, A., Valeeva, L. R., Chastukhina, I. B., Balaban, N. P., Shakirov, E. V., Greiner, R., & Sharipova, M. R. (2015). Novel glucose-1-phosphatase with high phytase activity and unusual metal ion activation from soil bacterium Pantoea sp. strain 3.5.1. Applied and Environmental Microbiology, 81, 6790–6799. https://doi.org/10.1128/AEM.01384-15.
Yan, J., Han, B., Gui, X., Wang, G., Xu, L., Yan, Y., Madzak, C., Pan, D., Wang, Y., Zha, G., & Jiao, L. (2018). Engineering Yarrowia lipolytica to simultaneously produce lipase and single cell protein from agroindustrial wastes for feed. Scientific Reports, 8, 758.
Groenewald, M., Boekhout, T., Neuvéglise, C., Gaillardin, C., van Dijck, P. W., & Wyss, M. (2014). Yarrowia lipolytica: safety assessment of an oleaginous yeast with a great industrial potential. Critical Reviews in Microbiology, 40(3), 187–206.
Madzak, C., Treton, B., & Blanchin-Roland, S. (2000). Strong hybrid promoters and integrative expression/secretion vectors for quasiconstitutive expression of heterologous proteins in the yeast Yarrowia lipolytica. Journal of Molecular Microbiology and Biotechnology, 2, 207–216.
Sambrook, J., & Russell, D. W. (2001). Molecular cloning: a laboratory manual. Cold: Cold Spring Harbor Laboratory Press.
Greiner, R. (2004). Degradation of myo-inositol hexakisphosphate by a phytate-degrading enzyme from Pantoea agglomerans. The Protein Journal, 23, 577–585.
Yao, X., Peijun, M., Premal, S., Antonis, R., & Yi, L. (2013). Non-optimal codon usage is a mechanism to achieve circadian clock conditionality. Nature, 495, 116–120.
Acknowledgments
This work was performed in accordance with the Russian Government Program of Competitive Growth of the Kazan Federal University and supported by the Russian Foundation for Basic Research (project no. 16-34-60191).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suleimanova, A.D., Troshagina, D.S., Madzak, C. et al. Heterologous Expression of Histidine Acid Phytase from Pantoea sp. 3.5.1 in Yarrowia lipolytica. BioNanoSci. 9, 44–47 (2019). https://doi.org/10.1007/s12668-018-0574-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-018-0574-8