Abstract
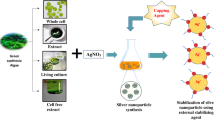
Properties of silver nanoparticles (AgNPs) are influenced by interactions and molecular structure of capping agents that stabilize them. Green synthesis of AgNPs using Lenzites betulina, a macrofungus with antioxidant properties, should be possible allowing the active metabolites or biomolecules to be incorporated in AgNP as capping molecules. Using surface plasmon resonance (SPR), the synthesis of AgNP using the aqueous extract of L. betulina was optimized. The purified L.betulina-capped AgNPs were then characterized and its antioxidant activity was compared with the raw L. betulina extract. The L. betulina-capped AgNPs exhibited SPR peak characteristic of AgNPs. Morphological analyses showed non-uniform spherical AgNP with visible intertwining capping agents engulfing the nanomaterial. FT-IR spectra revealed amide, carboxylate, and hydroxyl absorptions, confirming the role of some organic compounds as capping molecules. Extraction and complexation of the capping agent with SDS showed characteristic electronic excitation of phenylalanine along with tryptophan and tyrosine residues, suggesting the proteinaceous or polypeptide nature of the capping agent. The L. betulina-capped AgNPs were relatively stable (zeta potential − 26.7 mV), and the antioxidant activity was significantly enhanced compared to the raw extract. This is the first demonstration of L. betulina mushrooms in synthesizing AgNPs, the investigation of the proteinaceous capping biomolecules, and the enhanced effect of L. betulina-capped AgNPs in promoting stable radical reduction.

ᅟ







Similar content being viewed by others
References
Basavaraja, S., Balaji, S. D., Lagashetty, A., Rajasab, A. H., & Venkataraman, A. (2008). Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectum. Materials Research Bulletin, 43, 1164–1170. https://doi.org/10.1016/j.materresbull.2007.06.020.
Dipankar, C., & Murugan, S. (2012). The green synthesis, characterization, and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids and Surfaces. B, Biointerfaces, 98, 112–119. https://doi.org/10.1016/j.colsurfb.2012.04.006.
Cataldo, F., Ursini, O., & Angelini, G. (2013). A green synthesis of colloidal silver nanoparticles and their reaction with ozone. Eur Chem Bull, 2, 700–705. https://doi.org/10.17628/ecb.2013.2.700-705.
Abou El-Nour, K. M. M., Eftaiha, A., Al-Warthan, A., & Ammar, R. A. A. (2010). Synthesis and applications of silver nanoparticles. Arabian Journal of Chemistry, 3, 135–140. https://doi.org/10.1016/j.arabjc.2010.04.008.
Hussain, J., Kumar, S., Hashmi, A. A., & Khan, Z. (2011). Silver nanoparticles: preparation, characterization, and kinetics. Adv Mat Lett, 2, 188–194. https://doi.org/10.5185/amlett.2011.1206#sthash.kTIvbUae.dpuf.
Sudhakar, T., Nanda, A., Babu, S. G., Janani, S., Evans, M. D., & Markose, T. K. (2014). Synthesis of silver nanoparticles from edible mushroom and its antimicrobial activity against human pathogens. Int J PharmTech Res, 6, 1718–1723.
Philip, D. (2009). Biosynthesis of Au, Ag and Au-Ag nanoparticles using edible mushroom extract. Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 73, 374–381. https://doi.org/10.1016/j.saa.2009.02.037.
Hsu, T. H., Shiao, L. H., Hsieh, C., & Chang, D. M. (2002). A comparison of the chemical composition and bioactive ingredients of the Chinese medicinal mushroom DongChongXiaCao, its counterfeit and mimic, and fermented mycelium of Cordyceps sinensis. Food Chemistry, 78, 463–469. https://doi.org/10.1016/S0308-8146(02)00158-9.
Ballottin, D., Fulaz, S., Souza, M. L., Corio, P., Rodrigues, A. G., Souza, A. O., Gaspari, P. M., Gomes, A. F., Gozzo, F., & Tasic, L. (2016). Elucidating protein involvement in the stabilization of the biogenic silver nanoparticles. Nanoscale Research Letters, 11, 313. https://doi.org/10.1186/s11671-016-1538-y.
Chung, Y. C., Chen, I. H., & Chen, C. J. (2008). The surface modification of silver nanoparticles by phosphoryl disulfides for improved biocompatibility and intracellular uptake. Biomaterials, 29, 1807–1816. https://doi.org/10.1016/j.biomaterials.2007.12.032.
Lee, I. N., Yun, B. S., Cho, S. M., Kim, W. G., Kim, J. P., Ryoo, I. J., Koshino, H., & Yoo, I. D. (1996). Betulinans a and B, two benzoquinone compounds from Lenzites betulina. Journal of Natural Products, 59, 1090–1092. https://doi.org/10.1021/np960253z.
Liu, K., Wang, J. L., Wu, H. B., Wang, Q., Bi, K. L., & Song, Y. F. (2012). A new pyranone from Lenzites betulina. Chem Nat Comp, 48, 780–781. https://doi.org/10.1007/s10600-012-0380-4.
Liu, K., Wang, J. L., Zhao, L., & Wang, Q. (2014). Anticancer and antimicrobial activities and chemical composition of the birch Mazegill mushroom Lenzites betulina (higher basidiomycetes). Int J Med Mushrooms, 16, 327–337. https://doi.org/10.1615/IntJMedMushrooms.v16.i4.30.
Abdel-Aziz, M. S., Shaheen, M. S., El-Nekeety, A. A., & Abdel-Wahhab, M. A. (2014). Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract. Journal of Saudi Chemical Society, 18, 356–363. https://doi.org/10.1016/j.jscs.2013.09.011.
Khalil, M. M. H., Ismail, E. H., El-Baghdady, K. Z., & Mohamed, D. (2014). Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arabian Journal of Chemistry, 7, 1131–1139. https://doi.org/10.1016/j.arabjc.2013.04.007.
Fakoya, S., & Oloketuyi, S. F. (2012). Antimicrobial efficacy and phytochemical screening of mushrooms, Lenzites betulinus, and Coriolopsis gallica extracts. TAF Prev Med Bull, 11, 695–698. https://doi.org/10.5455/pmb.1-1327262044.
Merca, F. E., & Quizon, R. V. (2002). Isolation, purification and characterization of a lectin from Lenzites sp. Philipp Agric Sci, 85, 180–191.
Garidel, P., & Schott, H. (2006). Fourier-transform midinfrared spectroscopy for analysis and screening of liquid protein formulations. Bioprocess International, 48–55.
Ahmad, A., Mukherjee, P., Senapati, S., Mandal, D., Khan, M. I., Kumar, R., & Sastry, M. (2003). Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids and Surfaces. B, Biointerfaces, 28, 313–318. https://doi.org/10.1016/S0927-7765(02)00174-1.
Selvi, V. K., & Sivakumar, T. (2012). Isolation and characterization of silver nanoparticles from Fusarium oxysporum. International Journal of Current Microbiology and Applied Sciences, 1, 56–62.
Quester, K., Avalos-Borja, M., & Castro-Longoria, E. (2016). Controllable biosynthesis of small silver nanoparticles using fungal extract. J Biomater Nanobiotechnol, 7, 118–125. https://doi.org/10.4236/jbnb.2016.72013.
Jain, N., Bhargava, A., Rathi, M., Dilip, R. V., & Panwar, J. (2015). Removal of protein capping enhances the antibacterial efficiency of biosynthesized silver nanoparticles. PLoS One, 10. https://doi.org/10.1371/journal.pone.0134337.
Durán, N., Marcato, P. D., Alves, O. L., De Souza, G. I. H., & Esposito, E. (2005). Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. Journal of Nanbiotechnology, 3(8). https://doi.org/10.1186/1477-3155-3-8.
Phanjom, P., & Ahmed, G. (2015). Biosynthesis of silver nanoparticles by Aspergillus oryzae (MTCC no. 1846) and its characterizations. Nanoscience and Nanotechnology, 5, 14–21.
Shihab, R. N., Al-Kalifawi, E. J., & Al-Haidari, S. H. J. (2016). Environmental friendly synthesis of silver nanoparticles using leaf extract of Mureira tree (Azadirachta indica) cultivated in Iraq and efficacy the antimicrobial activity. Journal of Natural Science Research, 6, 47–56.
Umadevi, M., Shalini, S., & Bindhu, M. R. (2012). Synthesis of silver nanoparticles using D. carota extract. Advances in Natural Sciences: Nanoscience and Nanotechnology, 3(025008). https://doi.org/10.1088/2043-6262/3/2/025008.
Dhoondia, Z. H., & Chakraborty, H. (2012). Lactobacillus mediated synthesis of silver oxide nanoparticles. Nanomater Nanotechno, 2, 1–7. https://doi.org/10.5772/55741.
Xiao, F., Liu, H. G., & Lee, Y. I. (2008). Formation and characterization of two-dimensional arrays of silver oxide nanoparticles under Langmuir monolayers of n-hexadecyl dihydrogen phosphate. Bulletin of the Korean Chemical Society, 29, 2368–2372. https://doi.org/10.5012/bkcs.2008.29.12.2368.
Kumar, R., Roopan, S. M., Prabhakarn, A., Khanna, V. G., & Chakroborty, S. (2012). Agricultural waste Annona squamosa peel extract: biosynthesis of silver nanoparticles. Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 90, 173–176. https://doi.org/10.1016/j.saa.2012.01.029.
Mavani, K., & Shah, M. (2013). Synthesis of silver nanoparticles by using sodium borohydride as a reducing agent. Int J Eng Res Tech, 2(2), 1–5. https://doi.org/10.13140/2.1.3116.8648.
Sodha, K. H., Jadav, J. K., Gajera, H. P., & Rathod, K. J. (2015). Characterization of silver nanoparticles synthesized by different chemical reduction methods. International Journal of Pharma and Bio Sciences, 6, 199–208.
Yoosaf, K., Ipe, B. I., Suresh, C. H., & Thomas, K. G. (2007). In situ synthesis of metal nanoparticles and selective naked-eye detection of lead ions from aqueous media. Journal of Physical Chemistry C, 111, 12839–12847. https://doi.org/10.1021/jp073923q.
Mathew, J., Rathod, V., Singh, D., Singh, A. K., & Kulkarni, P. (2016). Antioxidant potential of AgNPs from the fungus Aspergillus pseudodeflectus by DPPH radical scavenging assay. Int J Pharm Pharm Sci Res, 6, 6–11.
Zieliǹski, H., & Kozɫowska, H. (2000). Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. Journal of Agricultural and Food Chemistry, 48, 2008–2016. https://doi.org/10.1021/jf990619o.
Ramakrishna, H., Murthy, S. S., Divya, R., MamathaRani, D. R., & Pandarunga, M. G. (2012). Hydroxy radical and DPPH scavenging activity of crude protein extract of Leucas linifolia: a folk medicinal plant. Asian J Plant Sci Res, 2, 30–35.
Ranjitham, A. M., Suja, R., Caroling, G., & Tiwari, S. (2013). In vitro evaluation of antioxidant, antimicrobial, anticancer activities and characterization of Brassica oleracea. var. Bortrytis. L synthesized silver nanoparticles. International Journal of Pharmacy and Pharmaceutical Sciences, 5, 239–251.
Hussin, F. R. M., Vitor, I. I. R. J. S., Joaquin, J. A. O., Clerigo, M. M., & Paano, A. M. C. (2016). Anti-hyperglycemic effects of aqueous Lenzites betulina extracts from the Philippines on the blood glucose levels of the ICR mice (Mus musculus). Asian Pac J Trop Biomed, 6, 155–158. https://doi.org/10.1016/j.apjtb.2015.04.013.
Funding
This work was supported by DLSU-URCO and National Research Council of the Philippines (NRCP)-DOST.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Highlights
• Active metabolite separation through the process of silver nanoparticle (AgNP) synthesis
• Synergistic effect of the proteinaceous capping biomolecules from L. betulina mushroom and AgNPs
• Antioxidant activity of L. betulina-capped AgNPs enhances stable radical reduction
Rights and permissions
About this article
Cite this article
Sytu, M.R.C., Camacho, D.H. Green Synthesis of Silver Nanoparticles (AgNPs) from Lenzites betulina and the Potential Synergistic Effect of AgNP and Capping Biomolecules in Enhancing Antioxidant Activity. BioNanoSci. 8, 835–844 (2018). https://doi.org/10.1007/s12668-018-0548-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-018-0548-x