Abstract
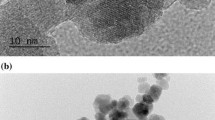
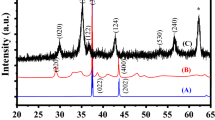
Optimal distribution of cerium oxide nanoparticles (CONPs) or nanoceria can have a significant impact on their cytotoxicity, cellular uptake, and radioprotection effects. In this study, two different distribution plans of CONPs were investigated. A scanner electron microscope (SEM) was used for chemical analysis and recording of CONP images. Using MTT assay, the non-toxic concentrations of nanoceria with two different distribution plans were determined in MRC-5 and MCF-7 cell lines. Nanoceria cellular uptake at 50, 150, and 250 μM with two different dispersion plans was determined by using the UV/VIS absorbance of cell culture medium after 24 h of incubation. In order to quantify radioprotection effect, cells treated with non-toxic concentrations of nanoceria were exposed to 10, 40, and 100 cGy of 6 MV photon beams. The diameter of the spherical CONPs was 29 nm. Energy dispersive spectroscopy analysis showed that the cerium element has the highest weight percentage in CONPs (97.9%). Accumulation rate of filtered and non-filtered suspension were determined as 0.3608 and 14.2708 μg/ml/h, respectively. The 70 and 110 μM concentration of sustained nanoceria suspension did not have any toxicity for MRC-5 and MCF-7 cells, respectively. In both cell lines, 50, 150, and 250 μM of filtered nanoceria had a significant uptake than the non-filtered nanoceria. A total of results showed that the 70 μM of nanoceria have a significant radioprotection on normal cells in the radiation dose of 40 and 100 cGy, while the highest cellular uptake of nanoceria occurred in cancer cells. The results suggest that using of stable distribution of CONPs for radiation protection could be a good choice, knowing that these nanostructures will have selective protection in normal cells.








Similar content being viewed by others
References
Najafi, M., Motevaseli, E., Shirazi, A., Geraily, G., Rezaeyan, A., Norouzi, F., et al. (2018). Mechanisms of inflammatory responses to radiation and normal tissues toxicity: Clinical implications. International Journal of Radiation Biology, 94, 335–356.
Yahyapour, R., Motevaseli, E., Rezaeyan, A., Abdollahi, H., Farhood, B., Cheki, M., et al. (2018). Reduction-oxidation (redox) system in radiation-induced normal tissue injury: molecular mechanisms and implications in radiation therapeutics. Clinical and Translational Oncology (in press).
Yahyapour, R., Motevaseli, E., Rezaeyan, A., Abdollahi, H., Farhood, B., Cheki, M., et al. (2018). Mechanisms of radiation bystander and non-targeted effects: Implications to radiation carcinogenesis and radiotherapy. Current Radiopharmaceuticals, 11, 34–45.
Wang, C., Blough, E., Dai, X., Olajide, O., Driscoll, H., Leidy, J. W., et al. (2016). Protective effects of cerium oxide nanoparticles on MC3T3-E1 osteoblastic cells exposed to X-ray irradiation. Cellular Physiology and Biochemistry, 38, 1510–1519.
Alavi, S. S., Dabbagh, S. T., Abbasi, M., & Mehrdad, R. (2017). Medical radiation workers’ knowledge, attitude, and practice to protect themselves against ionizing radiation in Tehran Province, Iran. Journal of Education and Health Promotion, 6, 58–64.
Velpula, N., Ugrappa, S., & Kodangal, S. (2017). A role of radioprotective agents in cancer therapeutics: a review. International Journal of Basic & Clinical Pharmacology, 2, 677–682.
Jafarpour, S. M., Safaei, M., Mohseni, M., Salimian, M., Aliasgharzadeh, A., & Fahood, B. (2018). The radioprotective effects of curcumin and trehalose against genetic damage caused by I-131. Indian Journal of Nuclear Medicine, 33, 99–104.
Safaei, M., Jafarpour, S. M., Mohseni, M., Salimian, M., Akbari, H., Karami, F., et al. (2018). Vitamins E and C prevent DNA double-strand breaks in peripheral lymphocytes exposed to radiations from iodine-131. Indian Journal of Nuclear Medicine, 33, 20–24.
Hirst, S. M., Karakoti, A. S., Tyler, R. D., Sriranganathan, N., Seal, S., & Reilly, C. M. (2009). Anti-inflammatory properties of cerium oxide nanoparticles. Small, 5, 2848–2856.
Nelson, B. C., Johnson, M. E., Walker, M. L., Riley, K. R., & Sims, C. M. (2016). Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants, 5, 15–35.
Vávrová, J., Řezáčová, M., & Pejchal, J. (2012). Fullerene nanoparticles and their anti-oxidative effects: a comparison to other radioprotective agents. Journal of Applied Biomedicine, 10, 1–8.
Niu, J., Wang, K., & Kolattukudy, P. E. (2011). Cerium oxide nanoparticles inhibits oxidative stress and nuclear factor-κB activation in H9c2 cardiomyocytes exposed to cigarette smoke extract. The Journal of Pharmacology and Experimental Therapeutics, 338, 53–61.
Chithrani, B. D., & Chan, W. C. (2007). Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Letters, 7, 1542–1550.
Alwan, A., MacLean, D. R., Riley, L. M., d'Espaignet, E. T., Mathers, C. D., Stevens, G. A., et al. (2010). Monitoring and surveillance of chronic non-communicable diseases: Progress and capacity in high-burden countries. Lancet, 376, 1861–1868.
Petri-Fink, A., Steitz, B., Finka, A., Salaklang, J., & Hofmann, H. (2008). Effect of cell media on polymer coated superparamagnetic iron oxide nanoparticles (SPIONs): colloidal stability, cytotoxicity, and cellular uptake studies. European Journal of Pharmaceutics and Biopharmaceutics, 68, 129–137.
Baker, C. H. (2013). Harnessing cerium oxide nanoparticles to protect normal tissue from radiation damage. Translational Cancer Research, 2, 343–358.
Valentin, J. (2007). The 2007 recommendations of the international commission on radiological protection. Oxford: Elsevier.
Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods, 65, 55–63.
Lee, C. L., Wan, C. C., & Wang, Y. Y. (2001). Synthesis of metal nanoparticles via self-regulated reduction by an alcohol surfactant. Advanced Functional Materials, 11, 344–347.
Sujana, M., Chattopadyay, K., & Anand, S. (2008). Characterization and optical properties of nano-ceria synthesized by surfactant-mediated precipitation technique in mixed solvent system. Applied Surface Science, 254, 7405–7409.
Karakoti, A. S., Singh, S., Kumar, A., Malinska, M., Kuchibhatla, S. V., Wozniak, K., et al. (2009). PEGylated nanoceria as radical scavenger with tunable redox chemistry. Journal of the American Chemical Society, 131, 14144–14145.
Zhang, P., Xie, C., Ma, Y., He, X., Zhang, Z., Ding, Y., et al. (2017). Shape-dependent transformation and translocation of ceria nanoparticles in cucumber plants. Environmental Science & Technology Letters, 4, 380–385.
Zook, J. M., MacCuspie, R. I., Locascio, L. E., Halter, M. D., & Elliott, J. T. (2011). Stable nanoparticle aggregates/agglomerates of different sizes and the effect of their size on hemolytic cytotoxicity. Nanotoxicology, 5, 517–530.
Lin, W., Huang, Y.-w., Zhou, X.-D., & Ma, Y. (2006). Toxicity of cerium oxide nanoparticles in human lung cancer cells. International Journal of Toxicology, 25, 451–457.
Pešić, M., Podolski-Renić, A., Stojković, S., Matović, B., Zmejkoski, D., Kojić, V., et al. (2015). Anti-cancer effects of cerium oxide nanoparticles and its intracellular redox activity. Chemico-Biological Interactions, 232, 85–93.
Rubio, L., Annangi, B., Vila, L., Hernández, A., & Marcos, R. (2016). Antioxidant and anti-genotoxic properties of cerium oxide nanoparticles in a pulmonary-like cell system. Archives of Toxicology, 90, 269–278.
Park, E.-J., Choi, J., Park, Y.-K., & Park, K. (2008). Oxidative stress induced by cerium oxide nanoparticles in cultured BEAS-2B cells. Toxicology, 245, 90–100.
García-Alonso, J., Rodriguez-Sanchez, N., Misra, S. K., Valsami-Jones, E., Croteau, M. N., Luoma, S. N., et al. (2014). Toxicity and accumulation of silver nanoparticles during development of the marine polychaete Platynereis dumerilii. The Science of the Total Environment, 476-477, 688–695.
Auffan, M., Rose, J., Wiesner, M. R., & Bottero, J.-Y. (2009). Chemical stability of metallic nanoparticles: A parameter controlling their potential cellular toxicity in vitro. Environmental Pollution, 157, 1127–1133.
Fröhlich, E. (2012). The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. International Journal of Nanomedicine, 7, 5577–5591.
Yokel, R. A., Unrine, J. M., Wu, P., Wang, B., & Grulke, E. A. (2014). Nanoceria biodistribution and retention in the rat after its intravenous administration are not greatly influenced by dosing schedule, dose, or particle shape. Environmental Science. Nano, 1, 549–560.
Kamalieva, R., Ishmukhametov, I., Batasheva, S., Rozhina, E., & Fakhrullin, R. (2018). Uptake of halloysite clay nanotubes by human cells: colourimetric viability tests and microscopy study. Nano-Structures & Nano-Objects, 15, 54–60.
Aljamali, N. M. (2015). Zetasizer technique in biochemistry. Biochemistry & Analytical Biochemistry, 4, 1–5.
Colon, J., Herrera, L., Smith, J., Patil, S., Komanski, C., Kupelian, P., et al. (2009). Protection from radiation-induced pneumonitis using cerium oxide nanoparticles. Nanomedicine, 5, 225–231.
Acknowledgements
This article was extracted from a master’s thesis in medical physics by the first author and it was supported by Shahid Sadoughi University of Medical Sciences, Yazd, Iran. We most especially acknowledge the friendly cooperation of the Shahid Remezanzadeh radiation oncology workers in Yazd, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Abdi Goushbolagh, N., Farhood, B., Astani, A. et al. Quantitative Cytotoxicity, Cellular Uptake and Radioprotection Effect of Cerium Oxide Nanoparticles in MRC-5 Normal Cells and MCF-7 Cancerous Cells. BioNanoSci. 8, 769–777 (2018). https://doi.org/10.1007/s12668-018-0538-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-018-0538-z