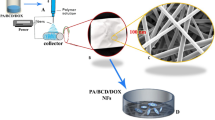
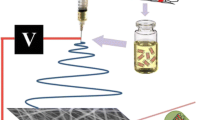
Abstract
Through the present research, for the first time, doxorubicin hydrochloride (DOX)-loaded poly (ε-caprolactone)-poly (ethylene glycol)-poly (ε-caprolactone) (PCL-PEG-PCL, PCEC) nanofibers were obtained by blending electrospinning method. The physicochemical characteristics of the DOX/PCEC nanofibers were fully evaluated. Nanofibers had homogeneous morphology with mean diameters ranging from 80 to 300 nm. The drug releasing behavior showed the faster release rate by increasing the drug content in nanofibers. The in vitro release profile and release kinetics from the DOX/PCEC nanofibers confirmed that the release profile followed Korsmeyer–Peppas model suggesting a Fickian diffusion as a major drug release mechanism.





Similar content being viewed by others
References
Zeighamian, V., et al. (2015). PNIPAAm-MAA nanoparticles as delivery vehicles for curcumin against MCF-7 breast cancer cells. Artificial Cells, Nanomedicine, and Biotechnology, 44, 1–8.
Salehi, R., et al. (2013). Stimuli-responsive nanofibers prepared from poly (N-isopropylacrylamide-acrylamide-vinylpyrrolidone) by electrospinning as an anticancer drug delivery. Designed Monomers and Polymers, 16(6), 515–527.
Xu, X., et al. (2008). The release behavior of doxorubicin hydrochloride from medicated fibers prepared by emulsion-electrospinning. European Journal of Pharmaceutics and Biopharmaceutics, 70(1), 165–170.
Zamani, M., Prabhakaran, M. P., & Ramakrishna, S. (2013). Advances in drug delivery via electrospun and electrosprayed nanomaterials. International Journal of Nanomedicine, 8, 2997.
Eatemadi, A., et al. (2015). Comparison, synthesis and evaluation of anticancer drug-loaded polymeric nanoparticles on breast cancer cell lines. Artificial Cells, Nanomedicine, and Biotechnology, 44, 1–10.
Feng, R., Song, Z., & Zhai, G. (2012). Preparation and in vivo pharmacokinetics of curcumin-loaded PCL-PEG-PCL triblock copolymeric nanoparticles. International Journal of Nanomedicine, 7, 4089.
Sridhar, R., et al. (2015). Electrosprayed nanoparticles and electrospun nanofibers based on natural materials: applications in tissue regeneration, drug delivery and pharmaceuticals. Chemical Society Reviews, 44(3), 790–814.
Ardeshirzadeh, B., et al. (2015). Controlled release of doxorubicin from electrospun PEO/chitosan/graphene oxide nanocomposite nanofibrous scaffolds. Materials Science and Engineering: C, 48, 384–390.
Sampath, M., et al. (2014). Curcumin loaded poly (lactic-co-glycolic) acid nanofiber for the treatment of carcinoma. Colloids and Surfaces B: Biointerfaces, 117, 128–134.
Valizadeh, A., et al. (2014) Preparation and characterization of novel electrospun poly (ϵ-caprolactone)-based nanofibrous scaffolds. Artificial Cells, Nanomedicine, and Biotechnology, 44, 1–6.
Agarwal, S., Greiner, A., & Wendorff, J. H. (2013). Functional materials by electrospinning of polymers. Progress in Polymer Science, 38(6), 963–991.
Hu, X., et al. (2014). Electrospinning of polymeric nanofibers for drug delivery applications. Journal of Controlled Release, 185, 12–21.
Qiu, K., et al. (2013). Doxorubicin-loaded electrospun poly (L-lactic acid)/mesoporous silica nanoparticles composite nanofibers for potential postsurgical cancer treatment. Journal of Materials Chemistry B, 1(36), 4601–4611.
Wu, S., et al. (2013). Adsorption properties of doxorubicin hydrochloride onto graphene oxide: equilibrium, kinetic and thermodynamic studies. Materials, 6(5), 2026–2042.
Mamaghani, P. Y., et al. (2015). Synthesis, characterization, and viscoelastic behavior of thermothickening poly (N-Isopropylacrylamide-Methacrylicacide-Vinylpyrrolidone) nanogels as an injectable biocompatible drug carrier. International Journal of Polymeric Materials and Polymeric Biomaterials, 64(2), 55–63.
Ta, H. T., et al. (2009). A chitosan–dipotassium orthophosphate hydrogel for the delivery of doxorubicin in the treatment of osteosarcoma. Biomaterials, 30(21), 3605–3613.
Salehi, R., Rasouli, S., & Hamishehkar, H. (2015). Smart thermo/pH responsive magnetic nanogels for the simultaneous delivery of doxorubicin and methotrexate. International Journal of Pharmaceutics, 487(1), 274–284.
Akbarzadeh, A., et al. (2012). Synthesis, characterization, and in vitro evaluation of novel polymer-coated magnetic nanoparticles for controlled delivery of doxorubicin. Nanotechnology, Science and Applications, 5, 13.
Yang, S. R., Lee, H. J., & Kim, J.-D. (2006). Histidine-conjugated poly (amino acid) derivatives for the novel endosomolytic delivery carrier of doxorubicin. Journal of Controlled Release, 114(1), 60–68.
Zarouni, M., et al. (2015). Biocompatible polymer coated paramagnetic nanoparticles for doxorubicin delivery: synthesis and anticancer effects against human breast cancer cells. International Journal of Polymeric Materials and Polymeric Biomaterials, 64(14), 718–726.
Gabizon, A., & Martin, F. (1997). Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Drugs, 54(4), 15–21.
Salehi, R., et al. (2014). Interaction, controlled release, and antitumor activity of doxorubicin hydrochloride from pH-sensitive P (NIPAAm-MAA-VP) nanofibrous scaffolds prepared by green electrospinning. International Journal of Polymeric Materials and Polymeric Biomaterials, 63(12), 609–619.
Liu, S., et al. (2013). Inhibition of orthotopic secondary hepatic carcinoma in mice by doxorubicin-loaded electrospun polylactide nanofibers. Journal of Materials Chemistry B, 1(1), 101–109.
Xu, B., et al. (2010). Amphiphilic biodegradable poly (ε-caprolactone)-poly (ethylene glycol)-poly (ε-caprolactone) triblock copolymers: synthesis, characterization and their use as drug carriers for folic acid. Polymer Bulletin, 64(6), 537–551.
Wei, X., et al. (2009). Biodegradable poly (ɛ-caprolactone)–poly (ethylene glycol) copolymers as drug delivery system. International Journal of Pharmaceutics, 381(1), 1–18.
Zhang, D., et al. (2012). Osteogenic differentiation of human placenta-derived mesenchymal stem cells (PMSCs) on electrospun nanofiber meshes. Cytotechnology, 64(6), 701–710.
Shen, X., et al. (2011). Electrospun diclofenac sodium loaded Eudragit® L 100-55 nanofibers for colon-targeted drug delivery. International Journal of Pharmaceutics, 408(1), 200–207.
Meinel, A. J., et al. (2012). Electrospun matrices for localized drug delivery: current technologies and selected biomedical applications. European Journal of Pharmaceutics and Biopharmaceutics, 81(1), 1–13.
Piras, A., et al. (2006). Development of diclofenac sodium releasing bio-erodible polymeric nanomats. Journal of Nanoscience and Nanotechnology, 6(9–10), 3310–3320.
Ngawhirunpat, T., et al. (2009). Development of meloxicam-loaded electrospun polyvinyl alcohol mats as a transdermal therapeutic agent. Pharmaceutical Development and Technology, 14(1), 73–82.
Sun, X.-Z., et al. (2013). Electrospun curcumin-loaded fibers with potential biomedical applications. Carbohydrate Polymers, 94(1), 147–153.
Ma, G., et al. (2011). Paclitaxel loaded electrospun porous nanofibers as mat potential application for chemotherapy against prostate cancer. Carbohydrate Polymers, 86(2), 505–512.
Acknowledgments
This work was financially supported by the “Department of Medical Nanotechnology, Faculty of Advanced Medical Science of Tabriz University” of Tabriz University of Medical Sciences. The authors thank the Stem Cell Research Center, Tabriz University of Medical Sciences, Tabriz, Iran, for all support provided.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Declaration of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bakhtiari, M., Salehi, R., Akbarzadeh, A. et al. Development of Novel Doxorubicin Loaded Biodegradable Polymeric Nanofibers as the Anticancer Drug Delivery Systems. BioNanoSci. 8, 60–66 (2018). https://doi.org/10.1007/s12668-017-0421-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-017-0421-3