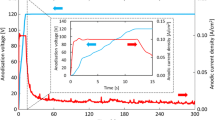
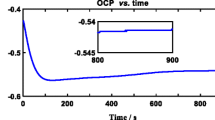
Abstract
Zirconium exhibited pseudo-passive behavior in fluorinated nitric acid (11.5 M HNO3 + 0.05 M NaF) as the current density measured from the electrochemical studies was several orders higher than the value in fluoride free nitric acid. Impedance studies on zirconium sample exposed in 11.5 M HNO3 for 240 h confirmed the formation of the passive film with high polarization resistance value and the calculated thickness of the film based on the capacitance value was about ~4.5 nm. On the other hand, in fluorinated nitric acid, the charge transfer resistance value associated with the zirconium dissolution process was dominant when compared to that of the film formation. Results of X-ray photoelectron spectroscopic investigations upheld the presence of ZrOF2 and ZrF4 and indicated that the protective oxide layer growth was restricted by the presence of fluoride ions.








Similar content being viewed by others
References
Baldev Raj and Kamachi Mudali U, Prog Nucl Energ 48 (2006) 283.
Bernard C, Mourox J P, Decours J, Demay R and Simonnet J, Proc Int Conf on Fuel Reprocessing and Waste Management-RECOD 91, Sendai, Japan (1991) p 570.
Jayaraj J, Thyagarajan K, Mallika C and Kamachi Mudali U, Nucl Technol 191 (2015) 58.
Jayaraj J, Krishnaveni P, Nanda Gopala Krishna D, Mallika C and Kamachi Mudali U, J Nucl Mater 473 (2016) 157.
Smith T and Hill G R, J Electrochem Soc 105 (1958) 117.
James W J, Custead W G and Straumanis M E, J Phys Chem 64 (1960) 286.
Straumanis M E, James W J and Custead W C, J Electrochem Soc 107 (1960) 502.
Van der Wall E M and Whitener E M, Ind Eng Chem 51 (1959) 51.
Goncalves Z and Munzel H, J Nucl Mater 170 (1990) 261.
Klein R, Corrosion 53 (1997) 327.
Sutter E M M, Hlawka F and Cornet A, Corrosion 46 (1990) 537.
Meyer R E, J Electrochem Soc 112 (1965) 684.
Prono J, Jaszay T, Caprani A and Frayret J P, J Appl Electrochem 25 (1995) 1031.
Fauvet P, Balbaud F, Robin R, Taran Q T, Mugnier A and Espinoux D, J Nucl Mater 375 (2008) 52.
Fontana M G, Corrosion Engineering, Tata McGraw-Hill Education Private Limited, Delhi (2005).
Ravi Shankar A and Kamachi Mudali U, Trans Indian Inst Metals 62 (2009) 545.
Kajimura H and Nagano H, Corros Sci 31 (1990) 261.
Lohrengel M M, Mater Sci Eng R 11 (1993) 243.
Kelly R G, Scully J R, Shoesmith D W, Buchheit R G, Electrochemical Techniques in Corrosion Science and Engineering, Marcel Dekker Inc., New York (2003).
Brug G J, Van Den Eeden A L G, Rehbach M S and Sluyters J H, J Electroanal Chem 176 (1984) 275.
Torres P C, Mesquita T J, Devos O, Tribollet B, Roche V and Nogueira R P, Electrochim Acta 72 (2012) 172.
Franceschetti D R and Macdonald J R, J Electroanal Chem 82 (1977) 271.
Torres P C, Keddam M and Nogueira R P, Electrochim Acta 54 (2008) 518.
Fasmin F, Praveen B V S and Ramanathan S, J Electrochem Soc 162 (2015) H604.
Armstrong R D and Edmonson K, Electrochim Acta 18 (1973) 937.
Sapra S, Li H, Wang Z and Suni I I, J Electrochem Soc 152 (2005) B193.
Diard J P, Gorrect B L and Montella C, J Electroanal Chem 432 (1997) 27.
Harrington D A, J Electroanal Chem 737 (2015) 30.
Harrington D A, J Electroanal Chem 449 (1998) 9.
He Z and Mansfeld F, Energ Environ Sci 2 (2009) 141.
Morant C, Sanz J M, Galan L, Soriano L and Rueda F, Surf Sci 218 (1989) 331.
Bosman H J M, Pijpers A P and Jaspers A W M A, J Catal 161 (1996) 551.
Sleigh C, Pijpers A P, Jaspers A, Coussens B and Meier R J, J Electron Spectrosc Relat Phenom 77 (1996) 41.
Jayaraj J, Ravishankar A and Kamachi Mudali U, Electrochim Acta 85 (2012) 210.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jayaraj, J., Nanda Gopala Krishna, D., Mallika, C. et al. Electrochemical Studies and XPS Analysis of the Surface of Zirconium-702 in Concentrated Nitric Acid With and Without Fluoride Ions. Trans Indian Inst Met 71, 521–531 (2018). https://doi.org/10.1007/s12666-017-1165-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-017-1165-z