Abstract
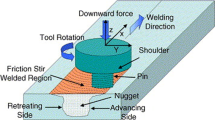
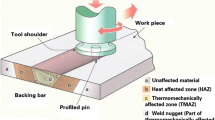
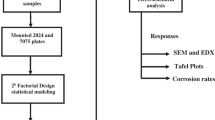
Aluminium alloy 5083, widely used in marine applications, undergoes accelerated corrosion in sea water due to the aggressive reaction of chloride ions with the secondary phase particles and other intermetallics present in the alloy matrix. The corrosion rate of the alloy is also influenced by the temperature difference between the alloy and its environment. Friction stir processing (FSP) is a recent solid state processing technique for improving the surface properties of metals and alloys. In this study, an attempt has been made to explore the possibility of improving the corrosion resistance of AA5083 by FSP. FSP trials were performed by varying the tool rotation speed, tool traverse speed and shoulder diameter of the tool, as per face centered central composite design. The corrosion potential and the corrosion rate of friction stir processed AA5083 was studied using potentiodynamic polarization studies, at three different temperatures. Mathematical models based on polynomial—radial basis function were developed and used to study the effect of process parameters on the corrosion potential and the corrosion rate of friction stir processed AA5083. FSP resulted in refinement of the grain structure, dispersion and partial dissolution of secondary phase particles in the matrix, which increased the corrosion resistance of the alloy.











Similar content being viewed by others
References
Bailey J C, Porter F C, Pearson A W, and Jarman R A, 4.1 - Aluminium and Aluminium Alloys, Butterworth-Heinemann, Oxford (1994).
Jafarzadeh K, Shahrabi T, Hadavi S M M, and Hosseini M G, Anti-Corros Methods Mater 56 (2009) 35.
Mishra R S, Ma Z, and Charit I, Mater Sci Eng A 341 (2003) 307.
Mishra R S, and Ma Z, Mater Sci Eng R 50 (2005) 1.
Cui G, Ma Z, and Li S, Acta Mater 57 (2009) 5718.
Ma Z, Metall Mater Trans A 39 (2008) 642.
Padmanaban R, V Ratna Kishore, and Balusamy V, Procedia Eng 97 (2014) 854. doi:10.1016/j.proeng.2014.12.360.
Padmanaban R, Balusamy V, and Nouranga K N, J Eng Sci Tech 10 (2015) 790.
Vaira Vignesh R, Padmanaban R, Arivarasu M, Karthick K, Sundar A A, Gokulachandran J, IOP Conference Series: Materials Science and Engineering, IOP Publishing (2016), p 012136.
Vignesh R V, Padmanaban R, Arivarasu M, Karthick K P, Sundar A A, and Gokulachandran J, in International Conference on Advances in Materials and Manufacturing Applications, Bengaluru IOP Conference Series: Materials Science and Engineering 149 (2016).
Smolej A, Klobčar D, Skaza B, Nagode A, Slaček E, Dragojević V, and Smolej S, Mater Sci Eng A 590 (2014) 239.
Fuller C B, and Mahoney M W, Metall Mater Trans A 37 (2006) 3605.
Santos T G, Lopes N, Machado M, Vilaça P, and Miranda R M, J Mater Process Technol 216 (2015) 375.
Sharma V, Gupta Y, Kumar B V M, and Prakash U, Mater Manuf Process 31 (2016) 1384.
Yuvaraj N, Aravindan S, Vipin, J Mater Res Technol 4 (2015) 398. doi:10.1016/j.jmrt.2015.02.006.
Johannes L B, Charit I, Mishra R S, and Verma R, Mater Sci Eng A 464 (2007) 351.
Hayashi J T, Menon S K, Su J Q, and McNelley T R, Key Eng Mater 443 (2010) 135.
Tan L, and Allen T R, Corros Sci 52 (2010) 548.
Rahimi H, Mozaffarinia R, Hojjati Najafabadi A, J Mater Sci Technol 29 (2013) 603.
Mars G F, Corrosion Engineering, Mc Graw-Hill Book Company, New York (2010).
Davis J R, Corrosion of Aluminum and Aluminum Alloys, A S M International, United States of America (1999).
Mishra R S, De P S, and Kumar N, Fundamental Physical Metallurgy Background for FSW/P, Springer International Publishing, Cham (2014).
Vaira Vignesh R, Padmanaban R, Arivarasu M, Thirumalini S, Gokulachandran J, Ram M S S S, IOP Conference Series: Materials Science and Engineering, IOP Publishing (2016), p 012208.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramalingam, V.V., Ramasamy, P. Modelling Corrosion Behavior of Friction Stir Processed Aluminium Alloy 5083 Using Polynomial: Radial Basis Function. Trans Indian Inst Met 70, 2575–2589 (2017). https://doi.org/10.1007/s12666-017-1110-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-017-1110-1