Abstract
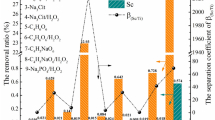
Extraction and stripping properties of Sc3+ were investigated by using a mixed extractant of Versatic Acid 10 (VA10) and Tri-n-butyl phosphate (TBP). The effect of adding TBP to VA10 was considered by analyzing the binding state of VA10 and TBP by FT-IR. Solvent extraction of scandium from an aqueous solution of Sc3+–Ti4+–Zr4+, which simulated waste water from the titanium oxide manufacturing process, the Bayers process and others, was performed, and the separation factor of Sc3+ over Ti4+ and Zr4+ was investigated. To clarify the operating conditions of a countercurrent multistage process for Sc3+, McCabe–Thiele analysis was applied to the extraction isotherm and stripping isotherm of Sc3+. The extraction pH of Sc3+ was found to shift to higher pH, and Sc3+ was stripped at a lower concentration of H2SO4 by adding TBP to VA10. Addition of TBP to VA10 suppresses the extraction of Sc3+ and promotes the stripping of Sc3+. IR spectra of the organic phase suggested that the dimeric portion of VA10 formed hydrogen bonds with TBP. The extractions of Sc3+, Ti4+ and Zr4+ were 77, 3 and 8%, respectively. The separation factors of these metal ions were βSc/Ti = 100 and βSc/Zr = 38 at pH4.6 on extraction with VA10 + TBP from aqueous solutionof Sc3+–Ti4+–Zr4+. Stripping efficiency of 100, 8 and 22% could be achieved for Sc3+, Ti4+ and Zr4+, respectively by using 0.5 mol/dm3 H2SO4. From these results, it was possible to separate Sc3+from impurities such as Ti4+ and Zr4+ by countercurrent multistage extraction, and Sc3+ was recovered by one-stage stripping with 0.5 mol/dm3 H2SO4. Extraction of Sc3+from the dilute aqueous solution containing 500 mg/dm3 of Sc3+reached 99% at the flow ratio (A/O) = 1.01 in the two-stage countercurrent extraction, and Sc3+ in the organic phase was stripped to attain a six-fold concentration by applying one-stage stripping with flow ratio of (O/A) = 6.00.









Similar content being viewed by others
References
Mii A, Iizuka T, J Illum Eng Int Jpn 77 (1993) 13.
Fujikawa S, Journal of Japan Institute of Light Minerals 49 (1999) 128.
Harada K, and Nakamura T (eds), CMC Substitution Material and Recycling of Rare Metals (2008) 1.
Iyatomi N, and Nanjo M, Tohoku University 45 (1989)66.
Mochida H, Resources processing 39 (1992) 161.
Sanematsu K, Hoshino M, and Watanabe Y, Shigen Chishitsu 62 (2012) 17.
Ito H, Senba K, Higashi K, and Yoshinaga M, J Min Metall Inst Jpn, 91 (1975)27.
Wang W, Pranlo Y, and Cheng C Y, Hydrometallurgy 108 (2011) 100.
Ochsenkühn M, Lyberopulu T H, and Parissakis G, Analytica Chimica Acta 315 (1995) 231.
Iyatomi N, and Mikami Y, Japanese Patent Disclosure (1995) H07-41976.
Isogawa C, Murayama N, and Shibata. J, Journal of Engineering Science and Technology 10 (2015) 78.
Shibata J, Ohtomo M, and Tanaka M, Kagaku KogakuRonbunshu 19 (1993) 214.
Ohmori H, Shibata J, Sano M, and Nishimura S, J Japan Ins. Metals 51 (1987) 645.
Palacios E G, and Monhemius A J, Hydrometallurgy 62 (2001) 135.
Levitskaia T G, Peterson J M, Campbell E L, Casella A D, Peterman D R, and Bryan SA, Industrial & Engineering Chemistry Research 52 (2013) 17607.
Toma, S, Uchihara T,and Kinjo A, Bulletin of Science &Engineering Division, University of Ryukyus. Mathematics & Natural Sciences 15 (1972) 102.
GaoH, Wu J, Zhu B, Xu G, Kexue Tnogbao 29 (1984) 883-887.
Reddy M L P, and Saji J, Mineral Processing and Extractive Metallurgy Review 23 (2002) 199.
Schrötteerová D, Nekovář P, and Mrnka M, Journal of Radioanalytical and Nuclear Chemistry 150 (1991) 325.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shibata, J., Murayama, N. Solvent Extraction of Scandium from the Waste Solution of TiO2 Production Process. Trans Indian Inst Met 70, 471–477 (2017). https://doi.org/10.1007/s12666-016-1008-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-016-1008-3