Abstract
Groundwater in crystalline basement is highly mineralized. The chemistry of bedrocks in crystalline basement of Ogbomosho in relation to chemical composition of groundwater in the area was, therefore, investigated employing a combined approach involving hydrogeological, geological mapping and geochemical method. A total of seventy five (75) water samples collected from wells; shallow and deep, bore within rock exposures (6) and other locations within the study area were analyzed for elemental composition using Inductive Coupled Plasma-Mass Spectrometry (ICP-MS). Physico-chemical parameters, temperature, electrical conductivity (EC), total dissolved solids (TDS) and acidity (pH), were measured in situ using HANNA HI9813-6 hand-held meter. Six rock samples representative of the main geological units of the study area were also collected and analyzed for major oxides and rare earth elements (REE) concentrations using ICP-MS. Thin sections of the rock samples were prepared and observed under petrological microscope for mineralogical compositions of the rocks. Acidity ions/TDS model of groundwater in the area was also established to determine the reactivity of the rock minerals and groundwater evolution pattern. The results of in-situ physico-chemical tests of the water samples indicate the temperature, EC, TDS and pH ranged within 26.6–31.7 °C, 72–1491 mS/cm, 36–747 ppm and 6.15–9.3, respectively. The petrographical analysis revealed biotite, quartz, potassic feldspar (microcline), perthite, albite, hornblende, plagioclase, myrmekite, topaz and muscovite. The major oxides, SiO2, Al2O3, Fe2O3, MgO, CaO, Na2O, K2O, TiO2, P2O5 and MnO, varied with median values of 72.21%, 14.99%, 1.17%, 0.375%, 2.245%, 4.215%, 3.46%, 0.16%, 0.045% and 0.015%. These indicate dorminance of SiO2 and Al2O3 suggesting acidic and metamorphic/acidic igneous rocks, respectively. The median percentage oxide compositions of the cations in rocks were, therefore, of the order: Na2O > K2O > CaO > MgO. The dissolved cations consequent upon weathering of the minerals were also of the order Na+ (26.9 mg/l) > K+ (4.69 mg/l) > Mg2+ (4.57 mg/l) > Ca2+ (4.23 mg/l) in groundwater based on their median values. These indicate solute concentration in groundwater is proportional to the reactivity of the bedrock minerals. The major anions, HCO3−, Cl− and NO3−, varied within 5.0–455 mg/l, 14.18–184.34 mg/l and 0.02–0.21 mg/l, respectively, in groundwater. The cross-plots of TDS against Ca2+, Na+, Cl− and HCO3− indicate the groundwater in the area is moderately to highly mineralized. The ionic reactivity based on pH ions/TDS plots indicated six-type models. The ionic concentration at low pH within 6.4–7.2 increases with pH for Type-1 (Ca2+), Type-5 (NO3−) and Type-6 (HCO3−,TDS), decreases for Type-2 (Mg2+, K+) but constant as pH increases for Type-4 (Cl−). At high pH, greater than 7.2 but less than 9, ionic concentration increases with pH for Type-1, Type-2 and Type-4, decreases with pH for Type-6 and constant for Type-5. However, the ionic concentration of Na+ (Type-3) momentarily increased, decreased and subsequently increased through low to high pH. These indicate varied degree of reactivity of the bedrock minerals and groundwater evolution pattern. Thus, the composition of dissolved ions in groundwater is controlled by weathering of Ca-feldspar (plagioclase), K-feldspar (orthoclase), Na-feldspar (albite) and biotite found in host rocks. The similarity in trend of cations in groundwater and their oxides in rock samples suggests the influences of local rock chemistry on the groundwater chemistry and hence, groundwater–rock interaction in the study area. The groundwater evolution pattern in the area depends on pH and reactivity of the ions produced from the weathering of the minerals. The chemical compositions of natural waters are, therefore, a direct indication of the geology of their catchment.


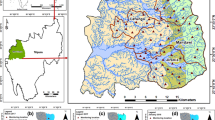
(Modified from Afolabi et al. 2013)












Similar content being viewed by others
References
Adabanija MA, Afolabi AO, Olatunbosun AT, Kolawole LL (2015) Integrated approach to investigation of occurrence and quality of groundwater in Ogbomoso North Southwestern, Nigeria. Environ Earth Sci 73(1):139–162
Afolabi OA, Kolawole LL, Abimbola AF, Olatunji AS, Ajibade OM (2013) Preliminary study of the geology and structural trends of lower Proterozoic Basement rocks in Ogbomoso SW, Nigeria. J Environ Earth Sci 3(8):82–95
Aghazadeh N, Mogaddam AA (2010) Assessment of Groundwater quality and its suitability for drinking and agricultural uses in the Oshavieh area, Northwest of Iran. J Environ Prot 1:30–40
Akwensioge M (2012) The influence of anthropogenic nitrate on groundwater quality in the Thaba Nehu area. MSc thesis, University of the Free State Bloemfontein.
Andre L, Franceschi P, Pouchan P, Atteia O (2005) Using geochemical data and modeling to enhance the understanding of groundwater flow in a regional deep aquifer, Aquitaine Basin, southwest of France. Hydrogeol J 33:50–62. https://doi.org/10.1016/jhydrol.2004.08.027
Anku YS, Banoeng YB, Asiedu DK, Asiedu SM (2009) Water quality analysis of groundwater in crystalline basement rocks, northern Ghana. Environ Geol 58:989–997
Al Yacoubi L, Bouchaou L, Jaillard E, Masrour M, Ait Brahim Y, El Mouden A, Schneider J, Reichert B (2017) Impact of rock-water interactions and recharge on water resources quality of the Agadir-Essaouira basin, southwestern Morocco. Arab J Geosci 10:169–184. https://doi.org/10.1007/s12517-017-2968-2
Azizullah A, Khattak MNK, Richter P, Hader DP (2011) Water pollution in Pakistan and its impact on public health—a review. Environ Int 37:479–497
Batabyal AK (2017) Hydrogeochemical processes and contaminants enrichment with special emphasis on fluoride in groundwater of Birbhum district, West Bengal India. Environ Earth Sci. 76:285–308. https://doi.org/10.1007/s12665-017-6584-y
Babiker LS, Mohamed AAM, Higama T, Kato K (2005) A GIS-based DRASTIC model for assessing aquifer vulnerability in Kakami Gahar Heights, Gifu Perfecture, Central Japan. Sci Total Environ 3(5):127–140
Banks D, Robins NS (2002) An introduction to groundwater in crystalline bedrock. Geological Survey of Norway, Trondheim
Beig MS, Lüttge A (2006) Albite dissolution kinetics as a function of distance from equilibrium: implications for natural feldspar weathering. Geochim Cosmochim Acta 70(6):1402–1420
Bell FG (2004) Engineering geology and construction. CRC Press, p 816. ISBN 10: 0415259398, 13: 9780415259392
Bottrell S, Hipkins EV, Lane JM, Zegos RA, Banks D, Frengstad BS (2019) Carbon-13 in groundwater from English and Norwegian crystalline rock aquifers: a tool for deducing the origin of alkalinity ? Sustain Water Resour Manag 5:267–287. https://doi.org/10.1007/s40899-017-0203-7
Boyton WV (1984) Cosmochemistry of the rare earth elements: meteorite studies. In: Burianek D, Brizova E, Cech S, Curda J, Furych V, Hanzil P, Kirchner K, Lysenko V, Henderson PE(eds) Rare element geochemistry: developments in geochemistry. Elsevier, Amsterdam, pp 63–114
Bucher K, Stober I (2010) Fluids in the upper continental crust. Geofluids 10:241–253
Chardon ES, Livens FR, Vaughan DJ (2006) Reactions of feldspar surfaces with aqueous solutions. Earth Sci Rev 78(1):1–26
Chilton PJ, Foster SSD (1995) Hydrological characterization and water-supply potential of basement aquifers in tropical Africa. Hydrogeol J 3:36–49
Cox KG, Bell JD, Pankhurst RJ (1979) The interpretation of igneous rocks. Springer, Dordrecht, p 450. https://doi.org/10.1007/978-94-017-3373-1
Crundwell FK (2015a) The mechanism of dissolution of the feldspars: part I. Dissolution at conditions far from equilibrium. Hydrometallurgy 151:151–162
Crundwell FK (2015b) The mechanism of dissolution of the feldspars: part II. Dissolution at conditions close to equilibrium. Hydrometallurgy 151:163–171
Edmunds WM, Shand P (2008) Natural groundwater quality. Blackwell Publishing, p 469. https://doi.org/10.1002/9781444300345
Frengstad B, Banks D, Siewers U (2001) The chemistry of Norwegian groundwaters:IV. The pH-dependence of element concentrations in crystalline bedrock groundwaters. Sci Total Environ 277:101–117
Foster SSD, Chilton PJ, Moencg M, Cardy F, Schiffler M (2000) Groundwater in rural development: facing the challenges of supply and resource sustainability (English). World Bank technical paper; no. WTP 463. The World Bank, Washington, D.C. http://documents.worldbank.org/curated/en/264071468766788418/Groundwater-in-rural-development-facing-the-challenges-of-supply-and-resource-sustainability Retrieved 5 Feb 2020
Frengstad B, Banks D, Skrede AAM, Krog JR, Siewers U, Strand T (2002) The hydrochemistry of crystalline bedrock groundwater in Norway. NUG-Bull 439:87–98
Galloway JN et al (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226
Gupta S, Mahato A, Roy P, Datta JK, Saha RN (2008) Geochemistry of groundwater, Burdwan district, West Bengal, India. Environ Geol 53:1271–1282. https://doi.org/10.1007/s00254-007-0725-7
Hasan SE, De Vivo B, Grasemann B, Stüwe K, Lastovicka J, Hasan SM, Yong C (2011) Environmental and engineering geology volume 1, EOLSS Publications, pp 434
Heim D (1990) Tone und Tonminerale [clays and clay minerals]. Enke, Stuttgart, p 157
Holloway JM, Dahlgren RA (2002) Nitrogen in rock: occurrences and biogeochemical implications. Glob Biogeochem Cycles 16(4):65-1-6517.1118
Holloway JM, Smith RL (2005) Nitrogen and carbon flow from rock to water: regulation through soil biogeochemical processes, Mokelumne River watershed, California, and Grand Valley, Colorado. J Geophys Res 110:F01010
Huneau F, Travi Y (2008) The Miocene aquifer of Valréas France. In: Edmunds WM, Piper Shand P (eds) Natural groundwater quality. Blackwell Publishing UK, pp 287–305. https://doi.org/10.1002/9781444300345
Iliopoulos V, Stamatis G, Stournaras G (2011) Marine and human activity effects on the groundwater quality of Thriassio Plain, Attica, Greece. In: Lambrakis N, Stournaras G, Katsanou K (eds) Advances in the research of aquatic environment. Environmental earth sciences. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-24076-8_48
Irfan M, Said M (2008) Hydrochemical characteristics and the effects of irrigation on groundwater quality in Harran Plain, GAP Project, Turkey. Environ Geol 54:183–196. https://doi.org/10.1007/s00254-007-0804-9
Irvine TN, Baragar WRA (1971) A guide to the chemical classification of the common volcanic rocks. Can J Earth Sci 8:523–548
Jalali M (2006) Chemical characteristics of groundwater in parts of mountainous region, Alvand, Ha madan, Iran. Environ Geol 51:433–446
Jalali M (2007) Assessment of the chemical components of Famenin groundwater, western Iran. Environ Geochem Health 29:357–374. https://doi.org/10.1007/s10653-006-9080-y
Jayasena HAH, Chandrajith R, Dissanayake CB (2008) Hydrogeochemistry of the groundwater flow system in a crystalline terrain: a study from the Kurunegala district, Sri Lanka. Environ Geol 55:723–730. https://doi.org/10.1007/s00254-007-1024-z
JMP (2008) Global water supply and sanitation 2008 report, joint monitoring programme WHO/UNICEF. World Health Organization, Geneva
Kampman N, Bickle M, Becker J, Assayag N, Chapman H (2009) Feldspar dissolution kinetics and Gibbs free energy dependence in a CO2-enriched groundwater system, Green River, Utah. Earth Planet Sci Lett 284(3–4):473–488
Kannan N, Joseph S (2009) Quality of groundwater in the shallow aquifers of a paddy dominated agricultural river basin, Kerala, India. World Acad Sci Eng Technol 52:475–493
Krishna Kumar S, Chandrasekar N, Seralathan P, Godson PS, Magesh NS (2011) Hydrogeochemical study of shallow carbonate aquifers, Rameswaram Island, India. Environ Monit Assess 184(7):4127–4139
Krishna Kumar S, Logeshkumaran A, Magesh NS, Godson PS, Chandrasekar N (2015) Hydro-geochemistry and application of water quality index (WQI) for groundwater quality assessment, Anna Nagar, part of Chennai City, Tamil Nadu, India. Appl Water Sci 5(4):335–343
Kumar M, Kumari K, Singh UK, Ramananthan AL (2009) Hydrogeochemical processes in the groundwater environment of Muktsar, Punjab: conventional graphical and multivariate statistical approach. Environ Geol 57:873–884
Lasaga AC, Luttge A (2001) Variation of crystal dissolution rate based on a dissolution stepwave model. Science 291(5512):2400–2404
Ludwig F (2011) Regional variation of chemical groundwater composition in Hessen Germany and its relation to the aquifer geology. Inaugural dissertation (PhD), Albert-Ludwigs-Universitat, Freibury, pp 175
MacDonald AM, Calow RC, Macdonald DMJ, Darling WG, ÓDochartaigh BE (2009) What impact will climate change have on rural groundwater supplies in Africa? Hydrol Sci J 54(4):690–703. https://doi.org/10.1623/hysj.54.4.690
MacDonald AM, Bonsor HC, Ó Dochartaigh BE, Taylor RG (2012) Quantitative maps of groundwater resources in Africa. Environ Res Lett 7(2):024009
Machiwal D, Cloutier V, Güler C, Kazakis N (2018) A review of GIS-integrated statistical techniques for groundwater quality evaluation and protection. Environ Earth Sci 77:681–687. https://doi.org/10.1007/s12665-018-7872-x
Maher K, Steefel CI, White AF, Stonestrom DA (2009) The role of reaction affinity and secondary minerals in regulating chemical weathering rates at the Santa Cruz Soil Chronosequence, California. Geochim Cosmochim Acta 73(10):2804–2831
Middlemost EAK (1985) Naming materials in the magma/igneous rock system. Earth Sci Rev 37:215–224
Oladunjoye MA, Adabanija MA, Oni AA (2013) Groundwater prospecting and exploration in a low potential hard rock aquifer: case study from Ogbomoso North South-western Nigeria. J Environ Earth Sci 3(14):84–102
Olasehinde PI (2015) Statistical Assessment of groundwater quality in Ogbomosho, Southwest Nigeria. Am J Min Metall 3(1):21–28. https://doi.org/10.12691/ajmm-3-1-4
Oluyide PO, Nwajide CS, Oni AO (1998) The geology of Ilorin area ministry of solid minerals development. Geol Surv Niger Bull 42:84
Peccerillo A, Taylor SR (1976) Geochemistry of Eocene calc-alkaline volcanic rocks from the Kastamonu area, Northern Turkey. Contrib Miner Petrol 58:63–81
Phillips ER (1974) Myrmekite—one hundred years later. Lithos 7(3):181–194. https://doi.org/10.1016/0024-4937(74),90029-2
Postma D, Kjøller C, Søgaard A, Condesso de Melo MT, Gaus I (2008) Geochemical modeling of processes controlling baseline composition of groundwater. In: Edmunds WM, Piper Shand P (eds) Natural groundwater quality. Blackwell Publishing, UK, pp 71–90
Rahaman MA (1988) Recent advances in the study of the basement complex of Nigeria. In: Oluyide PO, Mbonu WC, Ogezi AE, Egbuniwe IG, Ajibade AC and Umeji AC (eds.). Precambrian Geology of Nigeria, Geological Survey of Nigeria, pp 11–41
Prasanna MV, Chidambaram S, Shahul Hameed A, Srinivasamoorthy K (2011) Hydrogeochemical analysis and evaluation of groundwater quality in the Gadilam river basin, Tamil Nadu, India. J Earth Syst Sci 120:85–98
Rasolofonirina M, Randriamanivo LV, Andrianarilala MT, Andriambololona R (2004) Trace elements and physico-chemical quality of the well waters in Mahitsy, Province of Antananarivo, Madagascar. HEPMAD’04 International Conference, Madagascar, 27 September-1 October 2004
Ruizagudo E, King HE, Olópez LP, Putnis CV, Geisler T (2016) Control of silicate weathering by interface-coupled dissolution-precipitation processes at the mineral-solution interface. Geology 44(7):567–570
Rupert MG (2001) Calibration of the DRASTIC groundwater vulnerability mapping method. Ground Water 39:630–635
Sahoo PK, Kim K, Powell MA (2016) Managing groundwater nitrate contamination from livestock farms: implication for nitrate management guidelines. Curr Pollut Rep 2:178. https://doi.org/10.1007/s40726-016-0033-5
Schroeder HA (1960) Relation between mortality from cardiovascular disease and treated water supplies: variations in states and 163 largest municipalities of the United States. J Am Med Assoc 172:1902–1908. https://doi.org/10.1001/janna.1960.03020170028007
Sederholm JJ (1897) Übereine archaische Sedimentformation im südorestlichen Finlande: commission géologique de Finlande. Bulletin 6:254
Shukla S, Saxena A (2018) Global status of Nitrate contamination in groundwater: its occurrence, health impacts, and mitigation measures. In: Hussain CM (ed) Handbook of environmental materials management. https://doi.org/10.1007/978-3-319-58538-3_20-1
Sivasankar V, Darchen A, Omine K, Sakthivel R (2016) Fluoride: a world ubiquitous compound, its chemistry and ways of contamination. In: Sivansankar V. (ed.) Surface modified carbons as scavengers for fluoride from water, pp 29. https://doi.org/10.1007/978-3-319-40686-2_2
Srinivasa Gowd S (2005) Assessment of groundwater quality for drinking and irrigation purposes: a case study of Peddavanka watershed Anantapur District, Andhra Pradesh, India. Environ Geol 48:702–712. https://doi.org/10.1007/s00254-005-0009-z
Srinivasamoorthy K, Vijayaraghavan K, Vasanthavigar M, Sarma VS, Chidambaram S, Anandhan P (2010) Assessment of groundwater quality with special emphasis on fluoride contamination in crystalline bed rock aquifers of Mettur region, Tamilnadu, India. Arab J Geosci J Geosci 5:83–94. https://doi.org/10.1007/s12517-010-0162-x
Srinivasamoorthy K, Vasanthavigar M, Vijayaraghavan K, Sarathidasan R, Gopinath S (2013) Hydrochemistry of groundwater in a coastal region of Cuddalore district, Tamilnadu, India: implication for quality assessment. Arab J Geosci 6:441–454. https://doi.org/10.1007/s12517-011-0351-2
Stober I, Bucher K (1999) Deep groundwater in the crystalline basement of the Black Forest region. Appl. Geochem 14:237–254
Stober I, Zhu Y, Bucher K (2002) Water-rock reactions in a barite-fluorite underground mine, Black Forest (Germany). In: Stober I, Bucher K (eds) Water-rock interaction. Kluwer Academic Publishers, The Netherlands, pp 171–187
Tambekar DH, Neware BB (2012) Water quality index and multivariate analysis for groundwater quality assessment of villages of rural India. Sci Res Rep 2(3):229–235
Taylor SR, McLennan SM (1995) The geochemical evolution of the continental crust. Rev Geophys 33:241–265
Tyagi SK, Datta PS, Pruthi NK (2009) Hydrochemical appraisal of groundwater and its suitability in the intensive agricultural area of Muzaffarnagar district, Uttar Pradesh, India. Environ Geol 56:901–912. https://doi.org/10.1007/s00254-008-1190-7
Vasanthavigar M, Srinivasamoorthy K, Rajiv Ganthi R, Vijayaraghavan K, Sarma VS (2012) Characterisation and quality assessment of groundwater with a special emphasis on irrigation utility: Thirumanimuttar sub-basin, Tamil Nadu, India. Arab J Geosci 5:245–258. https://doi.org/10.1007/s12517-010-0190-6
Vernon RH (2004) A practical guide to rock microstructure. Cambridge University Press, Cambridge, p 594
Vinod PN, Chandramouli PN, Koch M (2015) Estimation of nitrate leaching in groundwater in an agriculturally used area in the state Karnataka, India, using existing model and GIS. Aquat Procedia 4:1047–1053
Walter J, Chesnaux R, Cloutier V, Gabourg D (2017) The influence of water/rock-water/clay interactions and mixing in the salinization processes of groundwater. J Hydrol Reg Stud 13:168–188. https://doi.org/10.1016/j.erjh.2017.07.004
Wen X, Wu Y, Su J, Zhang Y, Liu F (2005) Hydrochemical characteristics and salinity of groundwater in the Ejina Basin Northwestern China. Environ Geol 48:665–675. https://doi.org/10.1007/s00254-005-0001-7
White AF (2005) Natural weathering rates of silicate minerals. In: Drever JI (ed) Surface and groundwater, weathering, and soils, vol 5. Elsevier Science Paperback, pp 133–168
WHO (2011) Guidelines for drinking-water quality, 4th edn. World Health Organization, Geneva
Yuan G, Cao Y, Gluyas J, Jia Z (2017) Reactive transport modeling of coupled feldspar dissolution and secondary mineral precipitation and its implication for diagenetic interaction in sandstones. Geochim Cosmochim Acta 207:232–255
Yuan G, Cao Y, Schulz H-M, Hao F, Gluyas J, Liu K, Yang T, Wang Y, Xi K, Li F (2019) A review of feldspar alteration and its geological significance in sedimentary basins: from shallow aquifers to deep hydrocarbon reservoirs. Earth Sci Rev 191:114–140. https://doi.org/10.1016/j.earscirev.2019.02.004
Zhang L, Song X, Xia J, Yuan R, Zhang Y, Liu X, Han D (2011) Major element chemistry of the Huai River basin, China. Appl Geochem 26:293–300
Zhu C, Blum AE, Veblen DR, 2004. A new hypothesis for the slow feldspar dissolution in groundwater aquifers [C]. Geochim Cosmochim Acta 68(11): A148. The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, England: Pergamon-Elsevier Science Ltd.
Zhu C, Schwartz FW (2011) Hydrogeochemical processes and controls in water quality and water management. Elements 7:169–174
Zhu GF, Su YH, Feng Q (2008) The hydrochemical characteristics and evolution of groundwater and surface water in the Heihe River Basin, northwest China. J Hydrol 16:167–182
Zuhair K (2006) Characterization of surface water and groundwater in the Damascus Ghotta basin: hydrochemical and environmental isotopes approaches. Environ Geol 51:173–201. https://doi.org/10.1007/s00254-006-0316-z
Zuddas P (2010) Water-rock interaction processes seen through thermodymanics. Elements 6:305–308
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Adabanija, M.A., Afolabi, O.A. & Lawal, L. The influence of bedrocks on groundwater chemistry in a crystalline basement complex of southwestern Nigeria. Environ Earth Sci 79, 87 (2020). https://doi.org/10.1007/s12665-020-8822-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-020-8822-y