Abstract
Objective
The poor compliance to treatment of Helicobacter pylori-infected patients is well-known. We evaluated the efficacy of daily single-dose triple therapy as compared to conventional triple therapy on patient compliance and eradication of H. pylori infection.
Methods
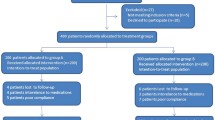
In the study group, 105 patients received esomeprazole 40 mg, tinidazole 1 g, and levofloxacin 500 mg once-daily for 14 days. One hundred and seven patients in the control group received lansoprazole 30 mg, amoxicillin 1 g, and clarithromycin 500 mg twice-daily for 14 days. Four weeks after completing therapy, urea breath test was performed to assess the eradication of H. pylori infection.
Results
The eradication rates by intention-to-treat analysis were 86% and 90.2% and by per-protocol analyses were 90.5% and 95.3% in the control and study groups, respectively, with no significant differences. Drug compliance was significantly better in the study group compared to the control group (p = 0.04). Overall, 44.7% of the patients in the study and 47.6% in the control groups had at least one adverse event. The most common adverse event was the dysgeusia in both the groups. The occurrence of diarrhea, nausea and vomiting was significantly higher in the control group and that of arthralgia was higher in the study group. The presence of periodontal disease and drug compliance was independently associated with treatment failure.
Conclusion
The use of single-dose PPI-based triple therapy improves drug compliance and eradication rate to standard PPI-based triple therapy. Presence of periodontal disease and drug compliance had negative influence on the eradication rate.
Trial Registration
NCT02711176

ᅟ

Similar content being viewed by others
References
O’Connor A, Gisbert J, McNamara D, O’Morain C. Treatment of Helicobacter pylori infection. Helicobacter. 2010;15:46–52.
Mansour-Ghanaei F, Yousefi Mashhour M, Joukar F, Sedigh M, Bagher-Zadeh AH, Jafarshad R. Prevalence of Helicobacter pylori infection among children in Rasht Northern Iran. Middle East J Dig Dis. 2009;1:84–8.
Malekzadeh R, Derakhshan MH, Malekzadeh Z. Gastric cancer in Iran: epidemiology and risk factors. Arch Iran Med. 2009;12:576–83.
Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24:1587–600.
Vakil N. Primary and secondary treatment for Helicobacter pylori in the United States. Rev Gastroenterol Disord. 2005;5:67–72.
Lee M, Kemp JA, Canning A, Egan C, Tataronis G, Farraye FA. A randomized controlled trial of an enhanced patient compliance program for Helicobacter pylori therapy. Arch Intern Med. 1999;159:2312–6.
Miner P, Katz PO, Chen Y, et al. Gastric acid control with esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole: a five-way crossover study. Am J Gastroenterol. 2003;98:2616–20.
Khademi F, Poursina F, Hosseini E, Akbari M, Ghasemian Safaei H. Helicobacter pylori in Iran: a systematic review on the antibiotic resistance. Iran J Basic Med Sci. 2015;18:2–7.
Jodlowski TZ, Lam S, Ashby CR Jr. Emerging therapies for the treatment of Helicobacter pylori infections. Ann Pharmacol Ther. 2008;42:1621–39.
Tursi A, Cammarota G, Montalto M, et al. Low-dose omeprazole plus clarithromycin and either tinidazole or amoxycillin for Helicobacter pylori infection. Aliment Pharmacol Ther. 1996;10:285–8.
Chu KM, Kwok KF, Law SY, Wong J. One-week once-daily triple therapy for helicobacter pylori--a pilot study. Hepatogastroenterology. 2000;47:1624–6.
Iacopini F, Crispino P, Paoluzi O, et al. One-week once-daily triple therapy with esomeprazole, levofloxacin and azithromycin compared to a standard therapy for Helicobacter pylori eradication. Dig Liver Dis. 2005;37:571–6.
Yee KCJ. Helicobacter pylori colonization of the oral cavity: a milestone discovery. World J Gastroenterol. 2016;14:641–8.
Zaric S, Bojic B, Jankovic L. Periodontal therapy improves gastric Helicobacter pylori eradication. J Dent Res. 2009;10:946–50.
Jia CL, Jiang GS, Li CH, Li CR. Effect of dental plaque control on infection of Helicobacter pylori in gastric mucosa. J Periodontol. 2009;80:1606–9.
Acknowledgements
We convey our gratitude to the Ilam University of Medical Sciences, Ilam, Iran, and the staff of Mostafa Khomeini Hospital.
Author information
Authors and Affiliations
Contributions
Z. V. and S. S. equally contributed to the conception/design of the research; S. S. contributed to the acquisition of the data; Z. V. contributed to the analysis and interpretation of the data. The authors drafted and critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Corresponding author
Ethics declarations
The study protocol was approved by the Institutional Ethics Committee.
Conflict of interest
SS and ZVS declare that they have no conflict of interest.
Ethical approval
The authors declare that the study was performed in a manner conforming to the Helsinki declaration of 1975, as revised in 2000 and 2008 concerning human and animal rights, and the authors followed the policy concerning informed consent as shown on Springer.com.
Disclaimer
The authors are solely responsible for the data and the content of the paper. In no way, the Honorary Editor-in-Chief, Editorial Board Members, or the printer/publishers are responsible for the results/ findings and content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shahbazi, S., Vahdat Shariatpanahi, Z. Comparison between daily single-dose triple therapy and conventional triple therapy on patient compliance and Helicobacter pylori eradication: A randomized controlled trial. Indian J Gastroenterol 37, 550–554 (2018). https://doi.org/10.1007/s12664-018-0916-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-018-0916-z