Abstract
Phosphorus (P) rich ash from biomass incineration is a potential promising alternative for non-renewable phosphate rock. This study considered the P recovery potential of poultry manure ash, sewage sludge ash and meat and bone meal ash through wet chemical extraction. X-ray diffraction analysis showed that these three ash types had a distinct P mineralogy. If inorganic acids were used for the extraction, the P extraction efficiency was not or only slightly affected by the P mineralogy. Contrarily, for the organic acids, alkaline extraction liquid and chelating agents considered, the P extraction efficiency was highly affected by the P mineralogy, and was also affected by the elemental composition of the ash and/or the chemical characteristics of the extraction liquids. Alkaline extraction liquids showed in general low heavy metal co-extraction, in contrast to the inorganic acids. From an economic point of view, of all extraction liquids considered, sulfuric acid was the most interesting to extract P from all three ash types. Oxalic acid could be a more sustainable option for P extraction from sewage sludge ash. In addition, extraction of poultry manure ash with ethylenediaminetetraacetic acid showed a relatively high P extraction efficiency combined with relatively low heavy metal co-extraction.
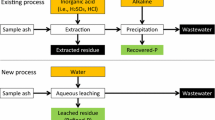
Graphic Abstract





Similar content being viewed by others
Availability of Data and Materials
Research data is stored and documented in a safe, secure and sustainable way and can be retrieved or accessed when needed.
Notes
Visual MINTEQ databases do not contain data on the chlorapatite (Ca5(PO4)3Cl) and fluorapatite (Ca5(PO4)3F) solubility. However, in the literature, it was found that for the ash types studied, apatite mainly occurs in the form of hydroxyapatite and only to a lesser extent in the form of chlorapatite and fluorapatite [19, 20, 26, 34]. Therefore, the conclusions in this paragraph are only based on the hydroxyapatite solubility.
Abbreviations
- MBM:
-
Meat and bone meal
- MBMA:
-
Meat and bone meal ash
- PM:
-
Poultry manure
- PMA:
-
Poultry manure ash
- SS:
-
Sewage sludge
- SSA:
-
Sewage sludge ash
References
U.S. Geological Survey (USGS): Phosphate rock. In: Mineral Commodity Summaries 2020, pp. 122–123. U.S. Geological Survey, Washington, D.C. (2020). https://doi.org/10.3133/mcs2020
Desmidt, E., Ghyselbrecht, K., Zhang, Y., Pinoy, L., Van der Bruggen, B., Verstraete, W., et al.: Global phosphorus scarcity and full-scale P-recovery techniques: a review. Crit. Rev. Environ. Sci. Technol. 45(4), 336–384 (2015). https://doi.org/10.1080/10643389.2013.866531
Mayer, B.K., Baker, L.A., Boyer, T.H., Drechsel, P., Gifford, M., Hanjra, M.A., et al.: Total value of phosphorus recovery. Environ. Sci. Technol. 50(13), 6606–6620 (2016). https://doi.org/10.1021/acs.est.6b01239
Cordell, D., White, S.: Sustainable phosphorus measures: strategies and technologies for achieving phosphorus security. Agronomy 3(1), 86–116 (2013). https://doi.org/10.3390/agronomy3010086
Takhim, M., Sonveaux, M., de Ruiter, R.: The Ecophos process: highest quality market products out of low-grade phosphate rock and sewage sludge ash. In: Ohtake, H., Tsuneda, S. (eds.) Phosphorus Recovery and Recycling, pp. 209–219. Springer, Singapore (2019). https://doi.org/10.1007/978-981-10-8031-9_14
Nättorp, A., Kabbe, C., Matsubae, K., Ohtake, H.: Development of phosphorus recycling in Europe and Japan. In: Ohtake, H., Tsuneda, S. (eds.) Phosphorus Recovery and Recycling, pp. 3–27. Springer, Singapore (2019). https://doi.org/10.1007/978-981-10-8031-9_1
Atienza-Martínez, M., Gea, G., Arauzo, J., Kersten, S.R.A., Kootstra, A.M.J.: Phosphorus recovery from sewage sludge char ash. Biomass Bioenerg. 65, 42–50 (2014). https://doi.org/10.1016/j.biombioe.2014.03.058
Donatello, S., Cheeseman, C.R.: Recycling and recovery routes for incinerated sewage sludge ash (ISSA): a review. Waste Manag. 33(11), 2328–2340 (2013). https://doi.org/10.1016/j.wasman.2013.05.024
Liang, S., Chen, H., Zeng, X., Li, Z., Yu, W., Xiao, K., et al.: A comparison between sulfuric acid and oxalic acid leaching with subsequent purification and precipitation for phosphorus recovery from sewage sludge incineration ash. Water Res. 159, 242–251 (2019). https://doi.org/10.1016/j.watres.2019.05.022
Adam, C., Peplinski, B., Michaelis, M., Kley, G., Simon, F.-G.: Thermochemical treatment of sewage sludge ashes for phosphorus recovery. Waste Manag. 29(3), 1122–1128 (2009). https://doi.org/10.1016/j.wasman.2008.09.011
Van de Velden, M., Dewil, R., Baeyens, J., Josson, L., Lanssens, P.: The distribution of heavy metals during fluidized bed combustion of sludge (FBSC). J. Hazard. Mater. 151(1), 96–102 (2008). https://doi.org/10.1016/j.jhazmat.2007.05.056
Kootstra, A.M.J., Brilman, D.W.F., Kersten, S.R.A.: Dissolution of phosphate from pig manure ash using organic and mineral acids. Waste Manag. 88, 141–146 (2019). https://doi.org/10.1016/j.wasman.2019.03.038
Langeveld, K.: Phosphorus recovery into fertilizers and industrial products by ICL in Europe. In: Ohtake, H., Tsuneda, S. (eds.) Phosphorus Recovery and Recycling, pp. 235–252. Springer, Singapore (2019). https://doi.org/10.1007/978-981-10-8031-9_16
Nusselder, S., de Graaff, L.G., Odegard, I.Y.R., Vandecasteele, C., Croezen, H.J.: Life cycle assessment and nutrient balance for five different treatment methods for poultry litter. J. Clean. Prod. 267, 121862 (2020). https://doi.org/10.1016/j.jclepro.2020.121862
Komiyama, T., Kobayashi, A., Yahagi, M.: The chemical characteristics of ashes from cattle, swine and poultry manure. J. Mater. Cycles Waste Manag. 15, 106–110 (2013). https://doi.org/10.1007/s10163-012-0089-2
Leng, L., Bogush, A.A., Roy, A., Stegemann, J.A.: Characterisation of ashes from waste biomass power plants and phosphorus recovery. Sci. Total Environ. 690, 573–583 (2019). https://doi.org/10.1016/j.scitotenv.2019.06.312
Billen, P., Costa, J., Van der Aa, L., Van Caneghem, J., Vandecasteele, C.: Electricity from poultry manure: a cleaner alternative to direct land application. J. Clean. Prod. 96, 467–475 (2015). https://doi.org/10.1016/j.jclepro.2014.04.016
Luyckx, L., de Leeuw, G.H.J., Van Caneghem, J.: Characterization of poultry litter ash in view of its valorization. Waste Biomass Valoriz. 11, 5333–5348 (2020). https://doi.org/10.1007/s12649-019-00750-6
Coutand, M., Cyr, M., Deydier, E., Guilet, R., Clastres, P.: Characteristics of industrial and laboratory meat and bone meal ashes and their potential applications. J. Hazard. Mater. 150(3), 522–532 (2008). https://doi.org/10.1016/j.jhazmat.2007.04.133
Deydier, E., Guilet, R., Sarda, S., Sharrock, P.: Physical and chemical characterisation of crude meat and bone meal combustion residue: “Waste or raw material?” J. Hazard. Mater. 121(1–3), 141–148 (2005). https://doi.org/10.1016/j.jhazmat.2005.02.003
Cohen, Y.: Phosphorus dissolution from ash of incinerated sewage sludge and animal carcasses using sulphuric acid. Environ. Technol. 30(11), 1215–1226 (2009). https://doi.org/10.1080/09593330903213879
Fang, L., Li, J., Guo, M.Z., Cheeseman, C.R., Tsang, D.C.W., Donatello, S., Poon, C.S.: Phosphorus recovery and leaching of trace elements from incinerated sewage sludge ash (ISSA). Chemosphere 193, 278–287 (2018). https://doi.org/10.1016/j.chemosphere.2017.11.023
Sarabèr, A.J.: Co-combustion and its impact on fly ash quality; Full-scale experiments. Fuel Process. Technol. 128, 68–82 (2014). https://doi.org/10.1016/j.fuproc.2014.06.026
European Sustainable Phosphorus Platform (ESPP). ESPP phosphorus fact sheet. Retrieved from https://phosphorusplatform.eu/images/download/ESPP-Phosphorus-fact-sheet-v21-4-19.pdf (2019)
Donatello, S., Tong, D., Cheeseman, C.R.: Production of technical grade phosphoric acid from incinerator sewage sludge ash (ISSA). Waste Manag. 30(8–9), 1634–1642 (2010). https://doi.org/10.1016/j.wasman.2010.04.009
Kaikake, K., Sekito, T., Dote, Y.: Phosphate recovery from phosphorus-rich solution obtained from chicken manure incineration ash. Waste Manag. 29(3), 1084–1088 (2009). https://doi.org/10.1016/j.wasman.2008.09.008
Ekpo, U., Ross, A.B., Camargo-Valero, M.A., Fletcher, L.A.: Influence of pH on hydrothermal treatment of swine manure: impact on extraction of nitrogen and phosphorus in process water. Bioresour. Technol. 214, 637–644 (2016). https://doi.org/10.1016/j.biortech.2016.05.012
Petzet, S., Peplinski, B., Cornel, P.: On wet chemical phosphorus recovery from sewage sludge ash by acidic or alkaline leaching and an optimized combination of both. Water Res. 46(12), 3769–3780 (2012). https://doi.org/10.1016/j.watres.2012.03.068
Darwish, M., Aris, A., Puteh, M.H., Jusoh, M.N.H., Kadir, A.A.: Waste bones ash as an alternative source of P for struvite precipitation. J. Environ. Manag. 203(2), 861–866 (2017). https://doi.org/10.1016/j.jenvman.2016.02.033
Franz, M.: Phosphate fertilizer from sewage sludge ash (SSA). Waste Manag. 28(10), 1809–1818 (2008). https://doi.org/10.1016/j.wasman.2007.08.011
Schaum, C., Cornel, P., Jardin, N.: Phosphorus recovery from sewage sludge ash—a wet chemical approach. In: Proceeding of the IWA Conference, pp. 583–590, June 24–27. Moncton, Canada (2007)
Xu, H., He, P., Gu, W., Wang, G., Shao, L.: Recovery of phosphorus as struvite from sewage sludge ash. J. Environ. Sci. 24(8), 1533–1538 (2012). https://doi.org/10.1016/S1001-0742(11)60969-8
Bogush, A.A., Stegemann, J.A., Williams, R., Wood, I.G.: Element speciation in UK biomass power plant residues based on composition, mineralogy, microstructure and leaching. Fuel 211, 712–725 (2018). https://doi.org/10.1016/j.fuel.2017.09.103
Kratz, S., Vogel, C., Adam, C.: Agronomic performance of P recycling fertilizers and methods to predict it: a review. Nutr. Cycl. Agroecosyst. 115, 1–39 (2019). https://doi.org/10.1007/s10705-019-10010-7
EMIS. CMA/2/II/A.3: Ontsluitingsmethode voor de bepaling van elementen in bodem, vaste en pasteuze materialen. Belgisch Staatsblad (2016)
European Committee for Standardization. EN 13656:2002 Characterization of waste—microwave assisted digestion with hydrofluoric (HF), nitric (HNO3) and hydrochloric (HCl) acid mixture for subsequent determination of elements (2002)
Azuara, M., Kersten, S.R.A., Kootstra, A.M.J.: Recycling phosphorus by fast pyrolysis of pig manure: concentration and extraction of phosphorus combined with formation of value-added pyrolysis products. Biomass Bioenerg. 49, 171–180 (2013). https://doi.org/10.1016/j.biombioe.2012.12.010
Luyckx, L.: Fosforherwinning uit pluimveemestverbrandingsassen en uit beendermeel. Masterproef ingediend tot het behalen van de graad van Master of Science in de Industriële Wetenschappen: Chemie: Sustainable Process and Materials Engineering, Faculteit Industriële Ingenieurswetenschappen, Campus Groep T, KU Leuven (2016)
Wang, Q., Li, J., Tang, P., Fang, L., Poon, C.S.: Sustainable reclamation of phosphorus from incinerated sewage sludge ash as value-added struvite by chemical extraction, purification and crystallization. J. Clean. Prod. 181, 717–725 (2018). https://doi.org/10.1016/j.jclepro.2018.01.254
Biswas, B.K., Inoue, K., Harada, H., Ohto, K., Kawakita, H.: Leaching of phosphorus from incinerated sewage sludge ash by means of acid extraction followed by adsorption on orange waste gel. J. Environ. Sci. 21(12), 1753–1760 (2009). https://doi.org/10.1016/S1001-0742(08)62484-5
Li, J., Chen, Z., Wang, Q., Fang, L., Xue, Q., Cheeseman, C.R., et al.: Change in re-use value of incinerated sewage sludge ash due to chemical extraction of phosphorus. Waste Manag. 74, 404–412 (2018). https://doi.org/10.1016/j.wasman.2018.01.007
Szögi, A.A., Vanotti, M.B., Hunt, P.G.: Phosphorus recovery from pig manure solids prior to land application. J. Environ. Manag. 157, 1–7 (2015). https://doi.org/10.1016/j.jenvman.2015.04.010
VLAREMA - Besluit van de Vlaamse Regering tot vaststelling van het Vlaams reglement betreffende het duurzaam beheer van materiaalkringlopen en afvalstoffen. Retrieved from https://navigator.emis.vito.be/mijn-navigator?woId=43991 (2018)
Kabbe, C., Rinck-Pfieffer, S.: Global compendium on phosphorus recovery from sewage/sludge/ash. Global Water Research Coalition (2019)
European Sustainable Phosphorus Platform (ESPP), German Phosphorus Platform (DPP), & Netherlands Nutrient Platform (NNP). Phosphorus recovery technology catalogue. Retrieved from https://phosphorusplatform.eu/images/download/ESPP-NNP-DPP_P-recovery_tech_catalogue_v_25_2_2020.pdf (2020)
Lee, M., Kim, D.-J.: Identification of phosphorus forms in sewage sludge ash during acid pre-treatment for phosphorus recovery by chemical fractionation and spectroscopy. J. Ind. Eng. Chem. 51, 64–70 (2017). https://doi.org/10.1016/j.jiec.2017.02.013
Fang, L., Li, J., Donatello, S., Cheeseman, C.R., Wang, Q., Poon, C.S., Tsang, D.C.W.: Recovery of phosphorus from incinerated sewage sludge ash by combined two-step extraction and selective precipitation. Chem. Eng. J. 348, 74–83 (2018). https://doi.org/10.1016/j.cej.2018.04.201
Li, J., Tsang, D.C.W., Wang, Q., Fang, L., Xue, Q., Poon, C.S.: Fate of metals before and after chemical extraction of incinerated sewage sludge ash. Chemosphere 186, 350–359 (2017). https://doi.org/10.1016/j.chemosphere.2017.08.012
Liu, J., Li, K., Wang, H., Zhu, M., Xu, H., Yan, H.: Self-assembly of hydroxyapatite nanostructures by microwave irradiation. Nanotechnology 16, 82–87 (2005). https://doi.org/10.1088/0957-4484/16/1/017
Peplinski, B., Adam, C., Michaelis, M., Kley, G., Emmerling, F., Simon, F.-G.: Reaction sequences in the thermochemical treatment of sewage sludge ashes revealed by X-ray powder diffraction—a contribution to the European project SUSAN. In: Zeitschrift für Kristallographie Supplemente, vol. 30, pp. 459–464. September 19–22. Warsaw, Poland (2009). https://doi.org/10.1524/zksu.2009.0068
Harvey, D.: Complexation titrations. Retrieved May 15, 2020, from https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Book%3A_Analytical_Chemistry_2.1_(Harvey)/09%3A_Titrimetric_Methods/9.03%3A_Complexation_Titrations (2020)
Deutsche Forschungsgemeinschaft (DFG): Nitrilotriacetic acid and its sodium salts. In: The MAK-Collection for Occupational Health and Safety. Wiley, Hoboken (2014). https://doi.org/10.1002/3527600418.mb13913vere4514
Nowak, B., Aschenbrenner, P., Winter, F.: Heavy metal removal from sewage sludge ash and municipal solid waste fly ash—a comparison. Fuel Process. Technol. 105, 195–201 (2013). https://doi.org/10.1016/j.fuproc.2011.06.027
Liu, J., Fu, J., Ning, X., Sun, S., Wang, Y., Xie, W., et al.: An experimental and thermodynamic equilibrium investigation of the Pb, Zn, Cr, Cu, Mn and Ni partitioning during sewage sludge incineration. J. Environ. Sci. 35, 43–54 (2015). https://doi.org/10.1016/j.jes.2015.01.027
Li, J., Xue, Q., Fang, L., Poon, C.S.: Characteristics and metal leachability of incinerated sewage sludge ash and air pollution control residues from Hong Kong evaluated by different methods. Waste Manag. 64, 161–170 (2017). https://doi.org/10.1016/j.wasman.2017.03.033
Zhang, Y., Cetin, B., Likos, W.J., Edil, T.B.: Impacts of pH on leaching potential of elements from MSW incineration fly ash. Fuel 184, 815–825 (2016). https://doi.org/10.1016/j.fuel.2016.07.089
Yang, T., Rao, S., Zhang, D., Wen, J., Liu, W., Chen, L., Zhang, X.: Leaching of low grade zinc oxide ores in nitrilotriacetic acid solutions. Hydrometallurgy 161, 107–111 (2016). https://doi.org/10.1016/j.hydromet.2016.01.024
Chembid. (n.d.). Prices for chemicals from different suppliers. Retrieved May 4, 2020, from https://www.chembid.com
Acknowledgements
This study was financially supported by BMC Moerdijk BV (Industrial Park M349, Middenweg 36a, 4782 PM Moerdijk, The Netherlands) and Fonds Wetenschappelijk Onderzoek Vlaanderen (FWO) (Lorien Luyckx is a SB PhD fellow at FWO, Project Number 1S08418N – 1S08420N). Furthermore, we want to thank BMC Moerdijk, Aquafin and Indaver for supplying the PMA, SSA and MBM samples. In addition, we want to thank Antoinette Deschuytere for carefully revising the manuscript.
Funding
This study was financially supported by BMC Moerdijk BV (Industrial Park M349, Middenweg 36a, 4782 PM Moerdijk, The Netherlands) and Fonds Wetenschappelijk Onderzoek Vlaanderen (FWO) (Project Number 1S08418N – 1S08420N).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Luyckx, L., Sousa Correia, D.S. & Van Caneghem, J. Linking Phosphorus Extraction from Different Types of Biomass Incineration Ash to Ash Mineralogy, Ash Composition and Chemical Characteristics of Various Types of Extraction Liquids. Waste Biomass Valor 12, 5235–5248 (2021). https://doi.org/10.1007/s12649-021-01368-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-021-01368-3