Abstract
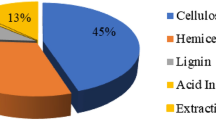
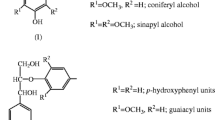
Lignin has aroused great interest in the scientific and economic areas because of its aromatic and complex nature. It can be used as a raw material for obtaining aromatic aldehydes but the great potential of lignin is not satisfactorily utilized. The interest is justified by the fact that, when its structure is spun off through pre-treatment processes, it produces some phenolic compounds of important industrial interest. These are bioactive compounds that can be used to promote benefits to human health, such as reducing the incidence of degenerative diseases like cancer and diabetes, as antioxidant, ant mutagenic activities, anti allergic, anti-inflammatory and antimicrobial effects. The present work aimed to the extraction and identification by UPLC-MS of biomolecules found in alkaline lignin hydrolysate from coffee husk. These compounds were classified as terpenes with relevant biological activities which make them interesting for pharmaceutical and cosmetic industries as discussed in literature.
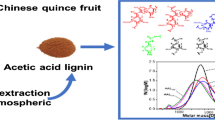
Graphic Abstract

Similar content being viewed by others
References
Souza, K.C.A., Abreu, H.S.: Biotechnology applied to the study of lignification. Floresta e Ambiente 14(1), 93–109 (2007)
García, A.A., Carril, E.P.-U.: Secondary plant metabolism. Reduca (Biologia) 2(3), 119–145 (2011)
Araújo, C.R., Garrido, C.V.S., Santos, J.M.G.M., Leal, S.C.S., Campos, L.M.A.: Study of the chemical and biological hydrolysis routes for the production of second generation ethanol from lignocellulosic residues. Student Seminar on Academic Production 12(1), (2013)
Saha, B.C.: Hemicellulose bioconversion. J. Ind. Microbiol. Biot. 30(5), 279–291 (2003)
Boopathy, R., Dawson, L.: Cellulosic ethanol production from sugarcane baggase without enzymatic saccharification. BioResources 3(2), 452–460 (2008)
Gonzaga, F.M.: Study of the influence of the alkaline/mechanical treatment on the mechanical properties of composites of short sisal/epoxy fibers, a undergraduate thesis presented at the Universidade Federal do Rio de Janeiro (2014)
Rabelo, S.C.: Evaluation and optimization of pre-treatments and enzymatic hydrolysis of sugarcane bagasse for the production of second generation ethanol, a doctoral thesis presented at the Universidade Estadual de Campinas (2010)
Bruice, P.Y.: Química Orgânica. Pearson Prentice Hall, São Paulo (2006)
Oliveira, F.D.C.: Oxidation of lignin from agroindustrial lignocellulosic residues to obtain aromatic chemical compounds with higher value-added, a doctoral thesis presented at the Universidade de São Paulo (2015)
Balasundram, N., Sundram, K., Samman, S.: Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem. 99(1), 191–203 (2006)
Sousa, C.M., Silva, H.R., Vieira, G.M., Ayres, M.C.C., Costa, C.L.S., Araújo, D.S., Cavalcante, L.C.D., Barros, E.D.S., Araújo, P.B.M., Brandão, M.S., Chaves, M.H.: Total phenols and antioxidant activity of five medicinal plants. Quím. Nova 30(2), 351–355 (2007)
Manach, C., Scalbert, A., Morand, C., Rémésy, C., Jiménez, L.: Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr 79(5), 727–747 (2004)
King, A., Young, G.: Characteristics and occurrence of phenolic phytochemicals. J. Am. Diet Assoc. 99(2), 213–218 (1999)
Rockenbach, I.I.: Phenolic compounds, fatty acids and antioxidant capacity of grape marc from vinification of red grapes (Vitis vinifera and Vitis labrusca), a master’s thesis presented at the Universidade Federal de Santa Catarina (2008)
Battestin, V., Matsuda, L.K., Macedo, G.A.: Sources and applications of tannins and tannases in food. Alimentos e Nutrição Araraquara 15(1), 63–72 (2008)
Brígida, A.I.S., Rosa, M.D.F.: Determination of Tannin content in the Coconut nucifera Bark. Proc. Interamerican Soc. Trop. Hortic. 47, 25–27 (2003)
Costa, C.T.C., Bevilaquia, C.M.L., Morais, S.M., Viera, S.L.: Tannins and their use in small ruminants. Rev. Bras. Plantas Med. 10(4), 108–116 (2008)
Almeida, N.F., Mori, F.A., Goulart, S.L., Mendes, L.M.: Study of reactivity of tannins of leaves and barks of Barbatimão Stryphnodendron adstringens (Mart.) Coville. Sci. Forest. 38(87), 401–408 (2010)
Durso, T. Sarrouh, B.: Deslignificação da casca de café: pré-tratamento de uma matéria-prima renovável promissora visando à obtenção de moléculas bioativas. 1ª. Ed. Novas Edições Acadêmicas. ISBN: 978620240633-8. p. 53. (2017)
Queiroz, S.C.N., Collins, C.H., Jardim, I.C.S.F.: Methods of extraction and/or concentration of compounds found in biological fluids for subsequent chromatographic determination, Quím. Nova 24(1), 68–76 (2001)
Engel, R., Kriz, G.S., Lampman, G.M., Pavia, D.L.: Química orgânica experimental – Técnicas em pequena escala, pp. 873–877. Cengage Learning, São Paulo (2012)
Chao, W., Lin, B.: Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian). Chin. Med. 5(17), 2–15 (2010)
Zhang, C.Y., Tan, B.K.: Mechanism of cardiovascular activity of Andrographispaniculata in the anaesthetized rat. J. Ethnopharmacol. 56, 97–101 (1997)
Zhang, C.Y., Tan, B.K.: Vasorelaxation of rat thoracic aorta caused by 14-deoxyandrographolide. Clin. Exp. Pharmacol. Physiol. 25, 424–429 (1998)
Yamaki, M., Bai, L., Kato, T., Inoue, K., Takagi, S.: Three dihydrophenanthropyrans from Bletilla Striata. Phytochemistry 32(2), 427–430 (1993)
Zhang, C.Y., Tan, B.K., Huang, W., Chen, Y., Jin, B., Chen, N., Ding, Z., Ding, X.: Antioxidant, antityrosinase and antitumor activity comparison: the potential utilization of fibrous root part of Bletilla striata (Thunb.) Reichb.f. PLoS ONE 8(2), e58004 (2013)
Lin, L.Z., Zhang, J.S., Chen, Z.L., Xu, R.S.: Isolation and identification of bruceaketolic acid and four other quassinoids. Acta Chim. Sinica 40, 73–78 (1982)
Lahrita, L., Moriai, K., Iwata, R., Kato, E.: Quassinoids in Brucea javanica are potent stimulators of lipolysis in adipocytes. Fitoterapia 137, 104250 (2019)
Zhang, Y., Liu, L., Xu, F., Shang, M., Liu, G., Cai, S.: Investigation of the in vivo metabolism of Sibirioside A and Angoroside C in rats by HPLC-ESI-IT-TOF-MS. Molecules 23, 2702–2716 (2018)
Zhang, L., Zhang, L., Zhang, Y.D.: Comparative study on the pharmacognosy and anti-inflammatory activities of Scrophularia buergeriana Miq. Var. tsinglingensis Tsoong and S. ninpoensis Hemsl. Northwest Pharm. J. 29, 264–267 (2014)
Bermejo, B.P., Diaz, L.A., Silvan, S.A., De Santos, G.Z., Fernandez, M.L., Sanz, G.A., Abad, M.J.: Effects of some iridoids from plant origin on arachidonic acid metabolism in cellular systems. Planta Med. 66, 324–328 (2000)
Ni, Z., Cai, X.Z., Huang, Y.P., Wang, D.J., Bian, H.M.: Effect of extracts of Scrophularia ningpoensis Hemsl. on hemorrheology, coagulation and fibrinolysis in rats. J. Chin. Microcirc. 3, 152–153 (2004)
Liu, G.L., Fu, P.Y., Wang, Z.Y., Xing, D.Y.: Effects of water extract of four Chinese herbal drugs on the binding of insulin with human erythroeyte insulin receptor. Chin. J. Integr. Trad. West. Med. 10, 606–607 (1991)
Huan-Li, W., Yan-Jing, L., Jun, C., Ping, L.: Triterpenoid saponins in roots of Achyranthese bidentata. Chin. J. Nat. Med 10(2), 98–101 (2012)
Castañeda, C.D.L. Revisión sobre los nortriterpenos aislados de la familia Simaroubaceae, y sobre un arbusto medicinal mexicano “chaparro amargoso” (Castela erecta subsp. texana). PhD thesis, Instituto de Química, U.N.A.M, México. 2005. P.96.
Singhal, S., Khare, M.P., Khare, A.: Cissogenin, a pregnane genin from Marsdenia tenacissima. Phytochem. 19(11), 2427–2430 (1980)
Weng, C., Chau, C., Hsieh, Y., Yang, S., Yen, G.: Lucidenic acid inhibits PMA-induced invasion of human hepatoma cells through inactivating MAPK/ERK signal transduction pathway and reducing binding activities of NF-kB and AP-1. Carcinogenesis 29(1), 147–156 (2008)
Hsu, C., Yen, G.: Chapter three: ganoderic acid and lucidenic acid (triterpenoid). Enzymes 36, 33–56 (2014)
Geng, C., Ma, Y., Zhang, X., Yao, S., Xue, D., Zhang, R., Chen, J.: Mulberrofuran G and isomulberrofuran G from Morus alba L. antihepatitis B virus activity and mass spectrometric fragmentation. J. Agric. Food Chem. 60, 8197–8202 (2012)
Wei, H., Zhu, J., Liu, X., Feng, W., Wang, Z., Yan, L.: Review of bioactive compounds from root barks of Morus plants (Sang-Bai-Pi) and their pharmacological effects. Cogent Chem. 2, 1212320 (2016)
Behl, T.: Herbal plants: a boon in the treatment of diabetic retinopathy. Pharmacologia. 6(1), 1–10 (2015)
Bhagya, N., Chandrashekar, K.R.: Tetrandrine: a molecule of wide bioactivity. Phytochemistry 125, 5–13 (2016)
Chen, Y.J.: Potential role of tetrandrine in cancer therapy. Acta Pharmacol. Sin. 23(12), 1102–1106 (2002)
Huang, Y.L., Cui, S.Y., Cui, X.Y., Cao, Q., Ding, H., Song, J.Z., Hu, X., Ye, H., Yu, B., Sheng, Z.F., Wang, Z.J., Zhang, Y.H.: Tetrandrine, an alkaloid from S. tetrandra exhibits anti-hypertensive and sleep-enhancing effects in SHR via different mechanisms. Phytomedicine 15(23), 1821–1829 (2016)
Chandrika, U.G., Prasad Kumarab, P.A.: Gotu kola (Centella asiatica): nutritional properties and plausible health benefits. Adv. Food Nutr. Res. 76, 125–157 (2015)
James, J.T., Dubery, I.A.: Pentacyclic triterpenoids from the medicinal herb, Centella asiatica (L.) urban. Molecules 14, 3922–3941 (2009)
Puttarak, P., Brantner, A., Panichayupakaranant, P.: Biological activities and stability of a standardized pentacyclic triterpene enriched Centella asiatica extract. Nat. Prod. Sci. 22(1), 20–24 (2016)
Xu, X., Wang, Y., Wei, Z., Wei, W., Zhao, P., Tong, B., Xia, Y., Dai, Y.: Madecassic acid, the contributor to the anti-colitis effect of madecassoside, enhances the shift of Th17 toward Treg cells via the PPARγ/AMPK/ACC1 pathway. Cell Death Dis. 8, e2723 (2017)
Bonté, F., Dumas, M., Chaudagne, C., Meybeck, A.: Comparative activity of asiaticoside and madecassoside on type I and III collagen synthesis by cultured human fibroblasts. Ann. Pharm Fr. 53(1), 38–42 (1995)
Guo-ping, P., Feng-chang, L.: Isolation and identification of diterpenes from Alisma orientalis Juzep. Acta Pharm. Sinica. 37(12), 950–954 (2002)
Hossain, M.E., Kim, G.M., Lee, S.K., Yang, C.J.: Growth performance, meat yield, oxidative stability, and fatty acid composition of meat from broilers fed diets supplemented with a medicinal plant and probiotics. Asian-Australas. J. Anim. Sci. 25, 1159–1168a (1168a)
Hossain, M.E., Ko, S.Y., Kim, G.M., Firman, J.D., Yang, C.J.: Evaluation of probiotic strains for development of fermented Alisma canaliculatum and their effects on broiler chickens. Poult. Sci. 91, 3121–3131 (2012)
Peng, G.P., Lou, F.C.: Isolation and identification of diterpenoids from Alisma orientalis. Acta Pharm. Sinica 37(12), 950–954 (2002)
Ikamo, H., Kawazoe, K., Izumi, K., Sato, Y., Tamaya, T.: Effects of crude herbal ingredients on intrauterineinfection in a rat model. Curr. Ther. Res. 59, 122–127 (1998)
Huang, Y.T., Huang, D.M., Chueh, S.C., Teng, C.M., Guh, J.H.: Alisol B acetate, a triterpene from Alismatisrhizoma, induces Bax nuclear translocation and apoptosis in human hormone-resistant prostate cancer PC-3cells. Cancer Lett. 231, 270–278 (2006)
Jang, M.K., Han, Y.R., Nam, J.S., Han, C.W., Kim, B.J., Jeong, H.S., Ha, K.T., Jung, M.H.: Protective effects of Alisma orientale extract against hepatic steatosis via inhibition of endoplasmic reticulum stress. Int. J. Mol. Sci. 16, 26151–26165 (2015)
Hu, T.M., Zhao, S.X.: The structures of oxofangchirine and stephenanthrine isolated from Stephania tetrandra S. Moore. Acta Pharm. Sin. 21, 29–34 (1986)
https://pubchem.ncbi.nlm.nih.gov/compound/10473975#section=2D-Structure
https://pubchem.ncbi.nlm.nih.gov/compound/45356919#section=Structures
Tian, T., Chen, H., Zhao, Y.: Traditional uses, phytochemistry, pharmacology, toxicology and quality control of Alisma orientale (Sam) Juzep: a review. J. Ethnopharmacol. 158, 373–387 (2014)
Santos, F.A., Queiróz, J.H., Colodette, J.L., Guimarães, V.M., Rezende, S.T.: Potencial da palha de cana-de-açúcar para produção de etanol. Quím. Nova 35(5), 1004–1010 (2012)
Acknowledgements
The authors would like to thank: CNPq, FAPEMIG and UFSJ for their financial support for the development of this study, and Waters Brazil for H-Class UPLC SM-FTN equipped analysis of the samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sarrouh, B., de Souza, R.O.A., da Silva Florindo, R.H. et al. Extraction and Identification of Biomolecules from Lignin Alkaline Hydrolysate from Coffee Husk. Waste Biomass Valor 12, 787–794 (2021). https://doi.org/10.1007/s12649-020-01021-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01021-5