Abstract
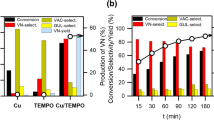
Prussian blue analogues (PBA), a class of metal-coordinated frameworks, are proposed in this study for aerobic oxidation of a lignin model compound, vanillyl alcohol (VAL), to the valuable product, vanillin (VN). While different metals and hexacyano-metalates are used to prepare various PBAs, the prototype PBA (Fe3[Fe(CN)6]2 abbreviated as “FeFe”) exhibited the highest catalytic activity towards VAL conversion to VN. The kinetics of VAL conversion is determined and the production of VN is also analyzed using the pseudo first order rate law. In addition, FeFe exhibits the highest catalytic activity to convert VAL to VN with the highest production and selectivity compared to the reported heterogeneous catalysts. FeFe can be also re-used to catalyze conversion of VAL to VN without significant activity loss. These features indicate that FeFe, as an easy-to-obtain and non-toxic pigment, is a promising catalyst for aerobic oxidation of VAL.







Similar content being viewed by others
References
Perlack, R.D., Wright, L.L., Turhollow, A.F., Graham, R.L., Stokes, B.J., Erbach, D.C.: Biomass as feedstock for a bioenergy and bioproducts industry: the technical feasibility of a billion-ton annual supply. DTIC Document (2005)
Behling, R., Valange, S., Chatel, G.: Heterogeneous catalytic oxidation for lignin valorization into valuable chemicals: what results? What limitations? What trends? Green Chem. 18, 1839–1854 (2016)
Lange, H., Decina, S., Crestini, C.: Oxidative upgrade of lignin—recent routes reviewed. Eur. Polym. J. 49, 1151–1173 (2013)
Azarpira, A., Ralph, J., Lu, F.: Catalytic alkaline oxidation of lignin and its model compounds: a pathway to aromatic biochemicals. BioEnergy Res. 7, 78–86 (2014)
Dai, J., Patti, A.F., Saito, K.: Recent developments in chemical degradation of lignin: catalytic oxidation and ionic liquids. Tetrahedron Lett. 57, 4945–4951 (2016)
Pan, J., Fu, J., Lu, X.: Microwave-assisted oxidative degradation of lignin model compounds with metal salts. Energy Fuels 29, 4503–4509 (2015)
Bulushev, D.A., Ross, J.R.H.: Catalysis for conversion of biomass to fuels via pyrolysis and gasification: a review. Catal. Today 171, 1–13 (2011)
Yokoyama, S. (ed.): Thermochemical conversion of biomass. In: Asia Biomass Handbook: A Guide for Biomass Production and Utilization, The Japan Institute of Energy, Tokyo (2008)
Jha, A., Patil, K.R., Rode, C.V.: Mixed Co–Mn oxide-catalysed selective aerobic oxidation of vanillyl alcohol to vanillin in base-free conditions. ChemPlusChem 78, 1384–1392 (2013)
Jha, A., Rode, C.V.: Highly selective liquid-phase aerobic oxidation of vanillyl alcohol to vanillin on cobalt oxide (Co3O4) nanoparticles. New J. Chem. 37, 2669–2674 (2013)
Saha, S., Hamid, S.B.A., Ali, T.H.: Catalytic evaluation on liquid phase oxidation of vanillyl alcohol using air and H2O2 over mesoporous Cu-Ti composite oxide. Appl. Surf. Sci. 394, 205–218 (2017)
Jha, A., Mhamane, D., Suryawanshi, A., Joshi, S.M., Shaikh, P., Biradar, N., Ogale, S., Rode, C.V.: Triple nanocomposites of CoMn2O4, Co3O4 and reduced graphene oxide for oxidation of aromatic alcohols. Catal. Sci. Technol. 4, 1771–1778 (2014)
Yuan, Z., Chen, S., Liu, B.: Nitrogen-doped reduced graphene oxide-supported Mn3O4: an efficient heterogeneous catalyst for the oxidation of vanillyl alcohol to vanillin. J. Mater. Sci. 52, 164–172 (2017)
Tarasov, A.L., Kustov, L.M., Bogolyubov, A.A., Kiselyov, A.S., Semenov, V.V.: New and efficient procedure for the oxidation of dioxybenzylic alcohols into aldehydes with Pt and Pd-based catalysts under flow reactor conditions. Appl. Catal. A 366, 227–231 (2009)
Ramana, S., Rao, B.G., Venkataswamy, P., Rangaswamy, A., Reddy, B.M.: Nanostructured Mn-doped ceria solid solutions for efficient oxidation of vanillyl alcohol. J. Mol. Catal. A 415, 113–121 (2016)
Behling, R., Chatel, G., Valange, S.: Sonochemical oxidation of vanillyl alcohol to vanillin in the presence of a cobalt oxide catalyst under mild conditions. Ultrason. Sonochem. 36, 27–35 (2017)
Fache, M., Boutevin, B., Caillol, S.: Vanillin production from lignin and its use as a renewable chemical. ACS Sustain. Chem. Eng. 4, 35–46 (2016)
Jiang, J.-A., Chen, C., Guo, Y., Liao, D.-H., Pan, X.-D., Ji, Y.-F.: A highly efficient approach to vanillin starting from 4-cresol. Green Chem. 16, 2807–2814 (2014)
Yepez, R., Garcia, S., Schachat, P., Sanchez-Sanchez, M., Gonzalez-Estefan, J.H., Gonzalez-Zamora, E., Ibarra, I.A., Aguilar-Pliego, J.: Catalytic activity of HKUST-1 in the oxidation of trans-ferulic acid to vanillin. New J. Chem. 39, 5112–5115 (2015)
Zakzeski, J., Bruijnincx, P.C.A., Jongerius, A.L., Weckhuysen, B.M.: The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 110, 3552–3599 (2010)
Makwana, V.D., Son, Y.-C., Howell, A.R., Suib, S.L.: The role of lattice oxygen in selective benzyl alcohol oxidation using OMS-2 catalyst: a kinetic and isotope-labeling study. J. Catal. 210, 46–52 (2002)
Kshirsagar, V.S., Garade, A.C., Patil, K.R., Shirai, M., Rode, C.V.: Liquid phase oxidation of p-cresol over cobalt saponite. Top. Catal. 52, 784–788 (2009)
Behera, G.C., Parida, K.M.: Liquid phase catalytic oxidation of benzyl alcohol to benzaldehyde over vanadium phosphate catalyst., Appl. Catal. A 413–414, 245–253 (2012)
Mishra, D.K., Dabbawala, A.A., Park, J.J., Jhung, S.H., Hwang, J.-S.: Selective hydrogenation of d-glucose to d-sorbitol over HY zeolite supported ruthenium nanoparticles catalysts. Catal. Today 232, 99–107 (2014)
Zakzeski, J., Jongerius, A.L., Weckhuysen, B.M.: Transition metal catalyzed oxidation of Alcell lignin, soda lignin, and lignin model compounds in ionic liquids. Green Chem. 12, 1225–1236 (2010)
Berrie, B.: Prussian Blue. National Gallery of Art, Washington (1997)
Questions and Answers on Prussian Blue, in: T.F.a.D.A. (FDA) (ed.), U.S. Food and Drug Administration, Silver Spring, MD (USA), 2003
WHO, WHO Model List of Essential Medicines, in: W.E.C.o.t.S.a.U.o.E. Medicines (ed.), WHO, Geneva, 2014
Torad, N.L., Hu, M., Imura, M., Naito, M., Yamauchi, Y.: Large Cs adsorption capability of nanostructured Prussian blue particles with high accessible surface areas. J. Mater. Chem. 22, 18261–18267 (2012)
M. M, S.P., Rd, O.N., R. R, L.-L.P., Jl, G.-M.: Prussian blue and analogues: biosensing applications in health care. In: Tiwari, A., Nordin, A.N. (eds.) Advanced Biomaterials and Biodevices. Wiley, Hoboken (2014)
Yue, Y., Binder, A.J., Guo, B., Zhang, Z., Qiao, Z.-A., Tian, C., Dai, S.: Mesoporous Prussian blue analogues: template-free synthesis and sodium-ion battery applications. Angew. Chem. Int. Ed. 53, 3134–3137 (2014)
Karadas, F., El-Faki, H., Deniz, E., Yavuz, C.T., Aparicio, S., Atilhan, M.: CO2 adsorption studies on Prussian blue analogues. Microporous Mesoporous Mater. 162, 91–97 (2012)
Liang, Y., Yi, C., Tricard, S., Fang, J., Zhao, J., Shen, W.: Prussian blue analogues as heterogeneous catalysts for epoxidation of styrene. RSC Adv. 5, 17993–17999 (2015)
Lin, K.-Y.A., Chen, B.-J., Chen, C.-K.: Evaluating Prussian blue analogues MII3[MIII(CN)6]2 (MII = Co, Cu, Fe, Mn, Ni; MIII = Co, Fe) as activators for peroxymonosulfate in water. RSC Adv. 6, 92923–92933 (2016)
Kaye, S.S., Long, J.R.: Hydrogen storage in the dehydrated Prussian blue analogues M3[Co(CN)6]2 (M = Mn, Fe, Co, Ni, Cu, Zn). J. Am. Chem. Soc. 127, 6506–6507 (2005)
Li, X., Liu, J., Rykov, A.I., Han, H., Jin, C., Liu, X., Wang, J.: Excellent photo-Fenton catalysts of Fe–Co Prussian blue analogues and their reaction mechanism study. Appl. Catal. B 179, 196–205 (2015)
Aksoy, M., Nune, S.V.K., Karadas, F.: A novel synthetic route for the preparation of an amorphous Co/Fe Prussian blue coordination compound with high electrocatalytic water oxidation activity. Inorg. Chem. 55, 4301–4307 (2016)
Pintado, S., Goberna-Ferrón, S., Escudero-Adán, E.C., Galán-Mascarós, J.R.: Fast and persistent electrocatalytic water oxidation by Co–Fe Prussian blue coordination polymers. J. Am. Chem. Soc. 135, 13270–13273 (2013)
Hu, M., Ishihara, S., Ariga, K., Imura, M., Yamauchi, Y.: Kinetically controlled crystallization for synthesis of monodispersed coordination polymer nanocubes and their self-assembly to periodic arrangements. Chemistry 19, 1882–1885 (2013)
Lin, K.-Y.A., Lai, H.-K., Chen, Z.-Y.: Selective generation of vanillin from catalytic oxidation of a lignin model compound using ZIF-derived carbon-supported cobalt nanocomposite. J. Taiwan Inst. Chem. Eng. 78, 337–343 (2017)
Elamathi, P., Kolli, M.K., Chandrasekar, G.: Catalytic oxidation of vanillyl alcohol using FeMCM-41 nanoporous tubular reactor. Int. J. Nanosci. 17, 1760010 (2018)
Adam, F., Chew, T.-S., Andas, J.: Liquid phase oxidation of acetophenone over rice husk silica vanadium catalyst. Chin. J. Catal. 33, 518–522 (2012)
Singh, A.P., Selvam, T.: Liquid phase oxidation of para-chlorotoluene to para-chlorobenzaldehyde using vanadium silicate molecular sieves. Appl. Catal. A 143, 111–124 (1996)
Lai, H.-K., Chou, Y.-Z., Lee, M.-H., Lin, K.-Y.A.: Coordination polymer-derived cobalt nanoparticle-embedded carbon nanocomposite as a magnetic multi-functional catalyst for energy generation and biomass conversion. Chem. Eng. J. 332, 717–726 (2018)
Shilpy, M., Ehsan, M.A., Ali, T.H., Hamid, S.B.A., Ali, M.E.: Performance of cobalt titanate towards H2O2 based catalytic oxidation of lignin model compound. RSC Adv. 5, 79644–79653 (2015)
Author information
Authors and Affiliations
Corresponding author
Additional information
Meng-Wei Zheng and Hong-Kai Lai have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zheng, MW., Lai, HK. & Lin, KY.A. Valorization of Vanillyl Alcohol by Pigments: Prussian Blue Analogue as a Highly-Effective Heterogeneous Catalyst for Aerobic Oxidation of Vanillyl Alcohol to Vanillin. Waste Biomass Valor 10, 2933–2942 (2019). https://doi.org/10.1007/s12649-018-0280-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-018-0280-3