Abstract
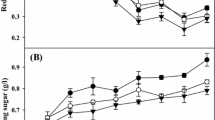
To find a multifunctional lignocellulolytic enzyme-producing strain, ten bacterial isolates from paper mill wastewater were tested for their carboxymethyl cellulose (CMC) hydrolytic ability. Bacillus sp. TPF-1, which exhibits the highest hydrolytic ability, was selected to produce lignocellulolytic enzymes using various biomass types as carbon sources. The highest CMCase (9.12 U/mL) and xylanase (102.55 U/mL) activities were obtained by green algae, and the maximum laccase activity (7037.28 U/L) was induced by Sargassum fusiforme. CMCase and xylanase showed the highest activities at 55 and 50 °C, respectively, with the same optimum pH of 5.4. The laccase exhibited optimum temperature of 40 °C and retained 60% more activity at 80 °C in extreme acid conditions (pH 2.2). To explore the multiple applications of these enzymes, crude enzymes induced by green algae were used to saccharify untreated algae. The reducing sugar produced by crude enzymes and commercial cellulase was 23 and 14% higher than that of the control, respectively, and it was 48% higher using crude enzymes with commercial cellulase (72 h). Additionally, the laccase induced by S. fusiforme was tested to decolorize two chemical dyes under an acidic condition (pH 2.2). The highest decolorization rates were 56.13 and 62.14% for Coomassie brilliant blue R-250 and Congo Red, respectively, in the presence of hydroxybenzotriazole monohydrate.






Similar content being viewed by others
References
Guo, H., Wu, Y., Hong, C., Chen, H., Chen, X., Zheng, B., Jiang, D., Qin, W.: Enhancing digestibility of Miscanthus using lignocellulolytic enzyme produced by Bacillus. Biores. Technol. 245, 1008–1015 (2017)
Sangrila, S., Maiti, T.: Cellulase production by bacteria: a review. Br. Microbiol. Res. J. 3(3), 235–258 (2013)
Maki, M., Leung, K.T., Qin, W.: The prospects of cellulase-producing bacteria for the bioconversion of lignocellulosic biomass. Int. J. Biol. Sci. 5(5), 500–516 (2009)
Alvira, P., Tomás-Pejó, E., Ballesteros, M., Negro, M.: Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Biores. Technol. 101(13), 4851–4861 (2010)
Otajevwo, F., Aluyi, H.: Cultural conditions necessary for optimal cellulase yield by cellulolytic bacterial organisms as they relate to residual sugars released in broth medium. Mod. Appl. Sci. 5(3), 141–151 (2011)
Daniel, G., Nilsson, T.: Developments in the study of soft rot and bacterial decay. In: Bruce, A., Palfreyman, J.W. (eds.) Forest Products Biotechnology, pp. 37–62. Taylor & Francis, London (1998)
Kim, H.-J., Lee, Y.-J., Gao, W., Chung, C.-H., Son, C.-W., Lee, J.-W.: Statistical optimization of fermentation conditions and comparison of their influences on production of cellulases by a psychrophilic marine bacterium, Psychrobacter aquimaris LBH-10 using orthogonal array method. Biotechnol. Bioprocess Eng. 16(3), 542–548 (2011)
Pandey, S., Singh, S., Yadav, A.N., Nain, L., Saxena, A.K.: Phylogenetic diversity and characterization of novel and efficient cellulase producing bacterial isolates from various extreme environments. Biosci. Biotechnol. Biochem. 77(7), 1474–1480 (2013)
Hung, K.-S., Liu, S.-M., Tzou, W.-S., Lin, F.-P., Pan, C.-L., Fang, T.-Y., Sun, K.-H., Tang, S.-J.: Characterization of a novel GH10 thermostable, halophilic xylanase from the marine bacterium Thermoanaerobacterium saccharolyticum NTOU1. Process Biochem. 46(6), 1257–1263 (2011)
Adlakha, N., Rajagopal, R., Kumar, S., Reddy, V.S., Yazdani, S.S.: Synthesis and characterization of chimeric proteins based on cellulase and xylanase from an insect-gut bacterium. Appl. Environ. Microbiol. 77(14), 4859–4866 (2011)
Fenel, F., Leisola, M., Jänis, J., Turunen, O.: A de novo designed N-terminal disulphide bridge stabilizes the Trichoderma reesei endo-1, 4-β-xylanase II. J. Biotechnol. 2(108), 137–143 (2004)
Verma, P., Madamwar, D.: Decolourization of synthetic dyes by a newly isolated strain of Serratia marcescens. World J. Microbiol. Biotechnol. 19(6), 615–618 (2003)
Givaudan, A., Effosse, A., Faure, D., Potier, P., Bouillant, M.-L., Bally, R.: Polyphenol oxidase in Azospirillum lipoferum isolated from rice rhizosphere: evidence for laccase activity in non-motile strains of Azospirillum lipoferum. FEMS Microbiol. Lett. 108(2), 205–210 (1993)
Ruijssenaars, H., Hartmans, S.: A cloned Bacillus halodurans multicopper oxidase exhibiting alkaline laccase activity. Appl. Microbiol. Biotechnol. 65(2), 177–182 (2004)
Giardina, P., Faraco, V., Pezzella, C., Piscitelli, A., Vanhulle, S., Sannia, G.: Laccases: a never-ending story. Cell. Mol. Life Sci. 67(3), 369–385 (2010)
Saratale, R.G., Saratale, G.D., Chang, J.-S., Govindwar, S.: Bacterial decolorization and degradation of azo dyes: a review. J. Taiwan Inst. Chem. Eng. 42(1), 138–157 (2011)
Vicente, A.I., Viña-Gonzalez, J., Santos-Moriano, P., Marquez-Alvarez, C., Ballesteros, A.O., Alcalde, M.: Evolved alkaline fungal laccase secreted by Saccharomyces cerevisiae as useful tool for the synthesis of C–N heteropolymeric dye. J. Mol. Catal. B 134, 323–330 (2016)
Claus, H.: Laccases of Botrytis cinerea. In: König, H., Unden, G., Fröhlich, J. (eds.) Biology of Microorganisms on Grapes, in Must and in Wine, pp. 339–356. Springer, New York (2017)
Lu, L., Wang, T.-N., Xu, T.-F., Wang, J.-Y., Wang, C.-L., Zhao, M.: Cloning and expression of thermo-alkali-stable laccase of Bacillus licheniformis in Pichia pastoris and its characterization. Biores. Technol. 134, 81–86 (2013)
Singh, G., Kaur, K., Puri, S., Sharma, P.: Critical factors affecting laccase-mediated biobleaching of pulp in paper industry. Appl. Microbiol. Biotechnol. 99(1), 155–164 (2015)
Rodriguez, E., Pickard, M.A., Vazquez-Duhalt, R.: Industrial dye decolorization by laccases from ligninolytic fungi. Curr. Microbiol. 38(1), 27–32 (1999)
Maki, M., Broere, M., Leung, K., Qin, W.: Characterization of some efficient cellulase producing bacteria isolated from paper mill sludges and organic fertilizers. Int. J. Biochem. Mol. Biol. 2(2), 146–154 (2011)
Guo, H., Chen, H., Fan, L., Linklater, A., Zheng, B., Jiang, D., Qin, W.: Enzymes produced by biomass-degrading bacteria can efficiently hydrolyze algal cell walls and facilitate lipid extraction. Renew. Energy 109, 195–201 (2017)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31(3), 426–428 (1959)
Laemmli, U.: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970)
Guo, H., Zheng, B., Jiang, D., Qin, W.: Overexpression of a laccase with dye decolorization activity from Bacillus sp. induced in Escherichia coli. J. Mol. Microbiol. Biotechnol. 27(4), 217–227 (2017)
Schinner, F., Von Mersi, W.: Xylanase-, CM-cellulase-and invertase activity in soil: an improved method. Soil Biol. Biochem. 22(4), 511–515 (1990)
Qinnghe, C., Xiaoyu, Y., Tiangui, N., Cheng, J., Qiugang, M.: The screening of culture condition and properties of xylanase by white-rot fungus Pleurotus ostreatus. Process Biochem. 39(11), 1561–1566 (2004)
Aa, K., Flengsrud, R., Lindahl, V., Tronsmo, A.: Characterization of production and enzyme properties of an endo-β-1, 4-glucanase from Bacillus subtilis CK-2 isolated from compost soil. Antonie Van Leeuwenhoek 66(4), 319–326 (1994)
Xu, F.: Effects of redox potential and hydroxide inhibition on the pH activity profile of fungal laccases. J. Biol. Chem. 272(2), 924–928 (1997)
Martins, L.O., Soares, C.M., Pereira, M.M., Teixeira, M., Costa, T., Jones, G.H., Henriques, A.O.: Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J. Biol. Chem. 277(21), 18849–18859 (2002)
Kocabas, A., Kocabas, D.S., Bolukbasi, U.B.: One-Step purification and characterization of a low molecular weight xylanase from Aspergillus terreus NRRL 1960. J. Appl. Biol. Sci. 5(2), 61–65 (2011)
Chaurasia, P.K., Yadav, R.S.S., Yadava, S.: Purification and characterization of yellow laccase from Trametes hirsuta MTCC-1171 and its application in synthesis of aromatic aldehydes. Process Biochem. 49(10), 1647–1655 (2014)
Haltrich, D., Nidetzky, B., Kulbe, K.D., Steiner, W., Župančič, S.: Production of fungal xylanases. Biores. Technol. 58(2), 137–161 (1996)
Panwar, D., Srivastava, P.K., Kapoor, M.: Production, extraction and characterization of alkaline xylanase from Bacillus sp. PKD-9 with potential for poultry feed. Biocatal. Agric. Biotechnol. 3(2), 118–125 (2014)
Amore, A., Parameswaran, B., Kumar, R., Birolo, L., Vinciguerra, R., Marcolongo, L., Ionata, E., La Cara, F., Pandey, A., Faraco, V.: Application of a new xylanase activity from Bacillus amyloliquefaciens XR44A in brewer’s spent grain saccharification. J. Chem. Technol. Biotechnol. 90(3), 573–581 (2015)
Han, S.J., Yoo, Y.J., Kang, H.S.: Characterization of a bifunctional cellulase and its structural gene The cel gene of Bacillus sp. D04 has exo-and endoglucanase activity. J. Biol. Chem. 270(43), 26012–26019 (1995)
Goyal, V., Mittal, A., Bhuwal, A.K., Singh, G., Yadav, A., Aggarwal, N.K.: Parametric optimization of cultural conditions for carboxymethyl cellulase production using pretreated rice straw by Bacillus sp. 313SI under stationary and shaking conditions. Biotechnol. Res. Int. 2014(12), 651839 (2014)
Ladeira, S.A., Cruz, E., Delatorre, A.B., Barbosa, J.B., Leal Martins, M.L.: Cellulase production by thermophilic Bacillus sp: SMIA-2 and its detergent compatibility. Electron. J. Biotechnol. 18(2), 110–115 (2015)
Ariffin, H., Abdullah, N., Kalsom, M.U., Shirai, Y., Hassan, M.: Production and characterisation of cellulase by Bacillus pumilus EB3. Int. J. Eng. Technol. 3(1), 47–53 (2006)
Breccia, J.D., Siñeriz, F., Baigorí, M.D., Castro, G.R., Hatti-Kaul, R.: Purification and characterization of a thermostable xylanase from Bacillus amyloliquefaciens. Enzyme Microb. Technol. 22(1), 42–49 (1998)
Lin, C., Shen, Z., Qin, W.: Characterization of xylanase and cellulase produced by a newly isolated Aspergillus fumigatus N2 and its efficient saccharification of barley straw. Appl. Biochem. Biotechnol. 182(2), 559–569 (2017)
Meunier-Goddik, L., Penner, M.H.: Enzyme-catalyzed saccharification of model celluloses in the presence of lignacious residues. J. Agric. Food Chem. 47(1), 346–351 (1999)
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y., Holtzapple, M., Ladisch, M.: Features of promising technologies for pretreatment of lignocellulosic biomass. Biores. Technol. 96(6), 673–686 (2005)
Burke, R., Cairney, J.: Laccases and other polyphenol oxidases in ecto-and ericoid mycorrhizal fungi. Mycorrhiza 12(3), 105–116 (2002)
Wang, W., Lu, J.-B., Wang, C., Wang, C.-S., Zhang, H.-H., Li, C.-Y., Qian, G.-Y.: Effects of Sargassum fusiforme polysaccharides on antioxidant activities and intestinal functions in mice. Int. J. Biol. Macromol. 58, 127–132 (2013)
Thanaraj, S., Seshadri, R.: Influence of polyphenol oxidase activity and polyphenol content of tea shoot on quality of black tea. J. Sci. Food Agric. 51(1), 57–69 (1990)
Sisler, E., Evans, H.: A comparison of chlorogenic acid and catechol as substrates for the polyphenol oxidase from tobacco and mushroom. Plant Physiol. 33(4), 255–257 (1958)
Chen, X., Nie, W., Fan, S., Zhang, J., Wang, Y., Lu, J., Jin, L.: A polysaccharide from Sargassum fusiforme protects against immunosuppression in cyclophosphamide-treated mice. Carbohyd. Polym. 90(2), 1114–1119 (2012)
Huang, X., Zhou, H., Zhang, H.: The effect of Sargassum fusiforme polysaccharide extracts on vibriosis resistance and immune activity of the shrimp, Fenneropenaeus chinensis. Fish Shellfish Immunol. 20(5), 750–757 (2006)
Chen, X., Nie, W., Yu, G., Li, Y., Hu, Y., Lu, J., Jin, L.: Antitumor and immunomodulatory activity of polysaccharides from Sargassum fusiforme. Food Chem. Toxicol. 50(3), 695–700 (2012)
Wu, X., Jiang, W., Lu, J., Yu, Y., Wu, B.: Analysis of the monosaccharide composition of water-soluble polysaccharides from Sargassum fusiforme by high performance liquid chromatography/electrospray ionisation mass spectrometry. Food Chem. 145, 976–983 (2014)
Baldrian, P.: Purification and characterization of laccase from the white-rot fungus Daedalea quercina and decolorization of synthetic dyes by the enzyme. Appl. Microbiol. Biotechnol. 63(5), 560–563 (2004)
Majeau, J.-A., Brar, S.K., Tyagi, R.D.: Laccases for removal of recalcitrant and emerging pollutants. Biores. Technol. 101(7), 2331–2350 (2010)
Hirose, J., Nasu, M., Yokoi, H.: Reaction of substituted phenols with thermostable laccase bound to Bacillus subtilis spores. Biotech. Lett. 25(19), 1609–1612 (2003)
Held, C., Kandelbauer, A., Schroeder, M., Cavaco-Paulo, A., Gübitz, G.M.: Biotransformation of phenolics with laccase containing bacterial spores. Environ. Chem. Lett. 3(2), 74–77 (2005)
Kawai, S., Nakagawa, M., Ohashi, H.: Degradation mechanisms of a nonphenolic β-O-4 lignin model dimer by Trametes versicolor laccase in the presence of 1-hydroxybenzotriazole. Enzyme Microb. Technol. 30(4), 482–489 (2002)
Acknowledgements
We acknowledge the financial support from BioFuelNet Canada, Lakehead University, National Special Fund for Forestry Scientific Research in the Public Interest of China (Grant No. 201504406), Natural Science Foundation of the Jiangsu Higher Education Institutions of China (Grant No. 15KJA220004), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest exits in the submission of this manuscript. We declare that we do not have any commercial or associative interest that represents a conflict of interest in the work submitted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, Y., Guo, H., Zhang, J. et al. Multiple Applications of Enzymes Induced by Algal Biomasses from a New Bacillus Isolate to Saccharify Algae and Degrade Chemical Dyes. Waste Biomass Valor 10, 2517–2526 (2019). https://doi.org/10.1007/s12649-018-0277-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-018-0277-y