Abstract
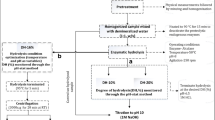
Salmon oil was extracted from cultured Atlantic salmon by-product mix (head, frame and viscera) through enzymatic extraction, with experimental combinations of different enzyme (Sea-B-Zyme L200 enzyme) concentrations (0.09–2.91%) and temperature levels (38–52 °C), generated and analyzed through Central Composite Rotatable design and response surface methodology respectively. The oil content obtained was between 13.09–19.18% of the total weight of the sample, where temperature level was observed as a more significant factor than enzyme concentration and highest oil yield was obtained at enzyme concentration of 2.5% and temperature level 50 °C. Salmon by-product oil predominantly contained mono-unsaturated fatty acids (52.49–54.27%), subsequently poly-unsaturated fatty acids (28.97–30.53%) and saturated fatty acids (14.95–17.91%). The extracted oil was also observed as a potential source of oleic acid, linoleic acid, palmitic acid, palmitoleic acid, stearic acid, vaccenic acid, gondoic acid, α-linoleic acid, DHA, DPA and EPA.


Similar content being viewed by others
References
Islam, M.S., Khan, S., Tanaka, M.: Waste loading in shrimp and fish processing effluents: potential source of hazards to the coastal and nearshore environments. Mar. Pollut. Bull. 49(1), 103–110 (2004)
Venugopal, V.: Chapter three—enzymes from seafood processing waste and their applications in seafood processing. In: Se-Kwon, K., Fidel, T. (eds.) Advances in Food and Nutrition Research. Vol 78. pp. 47–69. Academic Press, Cambridge (2016)
Jayasinghe, P., Hawboldt, K.: A review of bio-oils from waste biomass: Focus on fish processing waste. Renewable and sustainable energy reviews. 16(1), 798–821 (2012)
Etxabide, A., Leceta, I., Cabezudo, S., Guerrero, P., de la Caba, K.: Sustainable fish gelatin films: from food processing waste to compost. ACS Sustain. Chem. Eng. 4(9), 4626–4634 (2016)
Okazaki, E., Osako, K.: Isolation and characterization of acid-soluble collagen from the scales of marine fishes from Japan and Vietnam. Food chem. 149, 264–270 (2014)
Yahyaee, R., Ghobadian, B., Najafi, G.: Waste fish oil biodiesel as a source of renewable fuel in Iran. Renew. Sustain. Energy Rev. 17, 312–319 (2013)
Schiedt, K., Leuenberger, F.J., Vecchi, M.: Natural Occurrence of Enantiomeric and meso-Astaxanthin. 5. Ex wild salmon (Salmo salar and Oncorhynchus). Helv. Chim. Acta 64(2), 449–457 (1981)
Hudson, S., Smith, C.: Polysaccharides: chitin and chitosan chemistry and technology of their use as structural materials. In: Biopolymers from renewable resources pp. 96–118. Springer, Berlin (1998)
Arvanitoyannis, I.S., Kassaveti, A.: Fish industry waste: treatments, environmental impacts, current and potential uses. Int. J Food Sci. Technol. 43(4), 726–745 (2008)
Wu, T.H., Bechtel, P.J.: Salmon by-product storage and oil extraction. Food. Chem. 111(4), 868–871 (2008)
Mbatia, B., Adlercreutz, D., Adlercreutz, P., Mahadhy, A., Mulaa, F., Mattiasson, B.: Enzymatic oil extraction and positional analysis of ω-3 fatty acids in Nile perch and salmon heads. Process Biochem. 45(5), 815–819 (2010). doi:10.1016/j.procbio.2010.02.010
Linder, M., Fanni, J., Parmentier, M.: Proteolytic extraction of salmon oil and PUFA concentration by lipases. Mar. Biotechnol. 7(1), 70–76 (2005). doi:10.1007/s10126-004-0149-2
Taylor, S.L., King, J.W., List, G.R.: Determination of oil content in oilseeds by analytical supercritical fluid extraction. J. Am. Oil Chem. Soc. 70(4), 437–439 (1993)
Li, H., Pordesimo, L., Weiss, J.: High intensity ultrasound-assisted extraction of oil from soybeans. Food Res Int. 37(7), 731–738 (2004)
Lucchesi, M.E., Chemat, F., Smadja, J.: Solvent-free microwave extraction of essential oil from aromatic herbs: comparison with conventional hydro-distillation. J. Chromatogr. A 1043(2), 323–327 (2004)
Rosenthal, A., Pyle, D., Niranjan, K.: Aqueous and enzymatic processes for edible oil extraction. Enzyme Microb. Technol. 19(6), 402–420 (1996)
Johnson, L.A., Lusas, E.: Comparison of alternative solvents for oils extraction.. J. Am. Oil Chem. Soc. 60(2), 229–242 (1983)
Hara, A., Radin, N.S.: Lipid extraction of tissues with a low-toxicity solvent. Anal. Biochem. 90(1), 420–426 (1978)
Rosenthal, A., Pyle, D.L., Niranjan, K.: Aqueous and enzymatic processes for edible oil extraction. Enzyme Microb. Technol. 19(6), 402–420 (1996). doi:10.1016/S0141-0229(96)80004-F
Wang, L., Weller, C.L.: Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 17(6), 300–312 (2006)
Sherwin, E.: Oxidation and antioxidants in fat and oil processing. J. Am. Oil Chem. Soc. 55(11), 809–814 (1978)
Dominguez, H., Nunez, M., Lema, J.: Enzymatic pretreatment to enhance oil extraction from fruits and oilseeds: a review. Food chem. 49(3), 271–286 (1994)
Dave, D., Ramakrishnan, V.V., Pohling, J., Cheema, S.K., Trenholm, S., Manuel, H., Murphy, W.: Investigation on oil extraction methods and its influence on omega-3 content from cultured salmon. J. Food Process. Technol. (2014). doi:10.4172/2157-7110.1000401
Copeman, L.A., Parrish, C.C.: Lipids classes, fatty acids, and sterols in seafood from Gilbert Bay, Southern Labrador. J. Agric. Food Chem. 52(15), 4872–4881 (2004)
Parrish, C.C.: Lipids in marine ecosystems. ISRN Oceanogr. (2013). doi:10.5402/2013/604045
Hamoutene, D., Volkoff, H., Parrish, C., Samuelson, S., Mabrouk, G., Mansour, A., Mathieu, A., King, T., Lee, K.: Effect of produced water on innate immunity, feeding and antioxidant metabolism in Atlantic cod (Gadus morhua). In: Produced Water pp. 311–328. Springer, Berlin (2011)
Myers, R.H., Montgomery, D.C., Anderson-Cook, C.M.: Response surface methodology: process and product optimization using designed experiments. Wiley, Hoboken (2016)
Rubio-Rodríguez, N., Sara, M., Beltrán, S., Jaime, I., Sanz, M.T., Rovira, J.: Supercritical fluid extraction of fish oil from fish by-products: a comparison with other extraction methods. J. Food Eng. 109(2), 238–248 (2012)
Ohlson, J.: Processing effects on oil quality. J. Am. Oil Chem. Soc. 53(6), 299–301 (1976)
Balk, E.M., Lichtenstein, A.H., Chung, M., Kupelnick, B., Chew, P., Lau, J.: Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis. 189(1), 19–30 (2006)
Peet, M., Stokes, C.: Omega-3 fatty acids in the treatment of psychiatric disorders. Drugs 65(8), 1051–1059 (2005)
Rose, D.P., Connolly, J.M.: Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol Ther. 83(3), 217–244 (1999). doi:10.1016/S0163-7258(99)00026-1
Lopez-Huertas, E.: Health effects of oleic acid and long chain omega-3 fatty acids (EPA and DHA) enriched milks. A review of intervention studies. Pharmacol. Res. 61(3), 200–207 (2010). doi:10.1016/j.phrs.2009.10.007
Simopoulos, A.P.: Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 21(6), 495–505 (2002)
Samieri, C., Féart, C., Proust-Lima, C., Peuchant, E., Tzourio, C., Stapf, C., Berr, C., Barberger-Gateau, P.: Olive oil consumption, plasma oleic acid, and stroke incidence the three-city study. Neurology 77(5), 418–425 (2011)
Belury, M.A.: Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action 1. Annu. Rev. Nutr. 22(1), 505–531 (2002)
Ma, F., Hanna, M.A.: Biodiesel production: a review. Bioresour. Technol. 70(1), 1–15 (1999)
Imahara, H., Minami, E., Saka, S.: Thermodynamic study on cloud point of biodiesel with its fatty acid composition. Fuel 85(12–13), 1666–1670 (2006). doi:10.1016/j.fuel.2006.03.003
Field, C.J., Blewett, H.H., Proctor, S., Vine, D.: Human health benefits of vaccenic acid. Appl. Physiol. Nutr. Metab. 34(5), 979–991 (2009)
Zhang, J., Jia, S., Liu, Y., Wu, S., Ran, J.: Optimization of enzyme-assisted extraction of the Lycium barbarum polysaccharides using response surface methodology. Carbohydr. Polym. 86(2), 1089–1092 (2011). doi:10.1016/j.carbpol.2011.06.027
Abdulkarim, S., Long, K., Lai, O., Muhammad, S., Ghazali, H.: Some physico-chemical properties of Moringa oleifera seed oil extracted using solvent and aqueous enzymatic methods. Food. Chem. 93(2), 253–263 (2005)
Sengupta, R., Bhattacharyya, D.K.: Enzymatic extraction of mustard seed and rice bran. J. Am. Oil. Chem. Soc. 73(6), 687–692 (1996). doi:10.1007/bf02517941
Sharma, A., Gupta, M.N.: Ultrasonic pre-irradiation effect upon aqueous enzymatic oil extraction from almond and apricot seeds. Ultrason. Sonochem. 13(6), 529–534 (2006). doi:10.1016/j.ultsonch.2005.09.008
Latif, S., Diosady, L.L., Anwar, F.: Enzyme-assisted aqueous extraction of oil and protein from canola (Brassica napus L.) seeds. Eur. J. Lipid Sci. Technol. 110(10), 887–892 (2008)
Acknowledgements
Authors would like to thank RDC’s Ignite R&D program for providing the funding and continuous support to the project. Authors are grateful to Ms. Heather Manuel for her insight and her expertise in the project. Authors would also like to thank Ms. Sheila Trenholm (Laboratory Technologist, CASD, Marine Institute), Ms. Julia Pohling (Marine Biotechnologist, CASD, Marine Institute) and Jeanette Wells (Research Assistant, Aquatic Research Cluster, Memorial University of Newfoundland) for their expertise and support while conducting the research for their technical support while conducting the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Routray, W., Dave, D., Ramakrishnan, V.V. et al. Production of High Quality Fish Oil by Enzymatic Protein Hydrolysis from Cultured Atlantic Salmon By-Products: Investigation on Effect of Various Extraction Parameters Using Central Composite Rotatable Design. Waste Biomass Valor 9, 2003–2014 (2018). https://doi.org/10.1007/s12649-017-9998-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-9998-6