Abstract
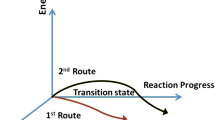
The multi-route reversible inhomogeneous chemical reaction involving seven chemical species is deliberated. To inspect the behavior of the common species, their activation energy and transition period have been measured before attaining equilibrium. It has been observed that some reaction routes complete their cycle before the others. The reason behind their completion is related to the species time period that remained involved in the reaction. Steady state behavior of chemical species is perceived. Graphical results are used to describe the physical aspects of measurements, while the difference is comparable in the tabulated form. The procedure is adopted by using MATLAB.






Similar content being viewed by others
References
G Marin and G S Yablonsky Kinetics of Chemical Reactions. (Wiley) (2011)
D Constales, G S Yablonsky, D R D’hooge, J W Thybaut and G B Marin Advanced Data Analysis and Modelling in Chemical Engineering. (Elsevier) (2016)
F Sultan, W A Khan, M Ali, M Shahzad, M Irfan and M Khan Pramana J. Phys. 92 21 (2019)
W A Khan, F Sultan, M Ali, M Shahzad, M Khan and M Irfan J. Braz. Soc. Mech. Sci. 41 4 (2019)
F Sultan, W A Khan, M Ali, M Shahzad, M Irfan and M Khan Pramana J. Phys. 92 16 (2019)
W A Khan, F Sultan, M Ali, M Shahzad, M Khan and M Irfan J. Braz. Soc. Mech. Sci. Eng. 41 4 (2019)
W A Khan, M Ali, F Sultan, M Shahzad, M Khan and M Irfan Pramana J. Phys. 92 16 (2019)
U Maas and S B Pope Combust. Flame 88 239 (1992)
A N Gorban and I V Karlin Chem. Eng. Sci. 58 4751 (2003)
E Chiavazzo, A N Gorban and I V Karlin Commun. Comput. Phys. 2 964 (2007)
E Chiavazzo and I V Karlin J. Comput. Phys. 227 5535 (2008)
A N Gorban and M Shahzad Entropy 13 966 (2011)
M Shahzad, S Rehman, R Bibi, H A Wahab, S Abdullah and S Ahmed Comput. Ecol. Softw. 5 254 (2015)
M Shahzad, H Arif, M Gulistan and M Sajid JCSP 37 207 (2015)
M Shahzad, F Sultan, I Haq, H A Wahab, M Naeem and F Haq The Nucleus 53 107 (2016)
M Shahzad, I Haq, F Sultan, A Wahab, F Faiz and G Rahman JCSP 39 828 (2017)
M Shahzad, F Sultan, I Haq, M Ali and W A Khan Pramana J. Phys. 92 64 (2019)
M Shahzad and F Sultan Advanced Chemical Kinetics. (InTech, Rijeka) (2018)
F Sultan, M Shahzad, M Ali and W A Khan AIP Adv. 9 015212 (2019)
M Shahzad, F Sultan, S I A Shah, M Ali, H A Khan and W A Khan J. Mol. Liq. 285 237 (2019)
M Shahzad, F Sultan, M Ali, W A Khan and M Irfan J. Mol. Liq. 284 265 (2019)
F Sultan, W A Khan, M Ali, M Shahzad, F Khan and M Waqas J. Mol. Liq. 288 111048 (2019)
J Horiuti Ann. N. Y. Acad. Sci. 213 5 (1973)
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sultan, F., Khan, W.A., Shahzad, M. et al. Activation energy characteristics of chemically reacting species in multi-route complex reaction mechanism. Indian J Phys 94, 1795–1802 (2020). https://doi.org/10.1007/s12648-019-01624-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12648-019-01624-2