Abstract
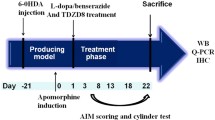
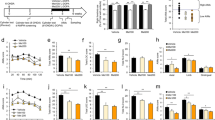
L-dopa is still considered as the gold standard therapy for Parkinson’s disease (PD); however, L-dopa-induced dyskinesia (LID) is a serious complication of long-term L-dopa treatment. The present study investigated the therapeutic potential of sitagliptin and liraglutide in comparisons with L-dopa against PD. In addition, their capacity to modulate L-dopa dose and/or side effects was investigated, too. Rats were injected with rotenone (3 mg/kg/day, s.c.) for 10 consecutive days to induce the experimental PD. The rotenone-treated rats were administered sitagliptin (30 mg/kg/day, p.o.) and liraglutide (50 μg/kg, s.c.) for 16 days either alone or together with L-dopa/carbidopa (50/25 mg/kg/day, i.p.). Scoring of LID was done on days 2, 4, 8, 12, and 16 in all L-dopa-treated groups. Twenty-four hours after the last administered dose of tested drugs, the behavior of rats in each group was screened by using the open-field test. Sitagliptin and liraglutide revealed marked attenuation of LID scores; in addition, they markedly increased animals’ motor performance. Moreover, they preserved substantia nigra pars compacta (SNpc) tyrosine hydroxylase (TH) and vesicular monoamine transporter 2–positive (VMAT2) cells with prominent increase of the striatal dopamine (DA) content. On the other hand, they significantly decreased nigral neuromelanin (NM)–positive cells, activated microglia, gliosis, and other pathological changes. In conclusion, sitagliptin and liraglutide could be a promising therapeutic challenger in PD, modifying L-dopa effect and/or allowing the use of L-dopa with fewer side effects.












Similar content being viewed by others
Abbreviations
- AIMs:
-
Abnormal involuntary movements
- CNS:
-
Central nervous system
- DA:
-
Dopamine
- GLP-1R:
-
Glucagon-like peptide-1 receptor
- GPCR:
-
G protein-coupled receptor
- LID:
-
L-dopa-induced dyskinesia
- NM:
-
Neuromelanin
- PD:
-
Parkinson’s disease
- SNpc:
-
Substantia nigra pars compacta
- TH:
-
Tyrosine hydroxylase
- VMAT2:
-
Vesicular monoamine transporter 2
References
Abdelsalam RM, Safar MM (2015) Neuroprotective effects of vildagliptin in rat rotenone Parkinson’s disease model: role of RAGE-NFkappaB and Nrf2-antioxidant signaling pathways. J Neurochem 133:700–707
Abdin AA, Sarhan NI (2011) Intervention of mitochondrial dysfunction-oxidative stress-dependent apoptosis as a possible neuroprotective mechanism of alpha-lipoic acid against rotenone-induced Parkinsonism and L-dopa toxicity. Neurosci Res 71:387–395
Abuirmeileh A, Harkavyi A, Rampersaud N, Lever R, Tadross JA, Bloom SR, Whitton PS (2012) Exendin-4 treatment enhances L-DOPA evoked release of striatal dopamine and decreases dyskinetic movements in the 6-hydoxydopamine lesioned rat. J Pharm Pharmacol 64:637–643
Alam M, Schmidt WJ (2002) Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res 136:317–324
Alam M, Mayerhofer A, Schmidt WJ (2004) The neurobehavioral changes induced by bilateral rotenone lesion in medial forebrain bundle of rats are reversed by L-DOPA. Behav Brain Res 151:117–124
Alves G, Forsaa EB, Pedersen KF, Dreetz Gjerstad M, Larsen JP (2008) Epidemiology of Parkinson’s disease. J Neurol 255(Suppl 5):18–32
Athauda D, Foltynie T (2016) The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson's disease: mechanisms of action. Drug Discov Today 21:802–818
Badawi GA, Abd El Fattah MA, Zaki HF, El Sayed MI (2017) Sitagliptin and liraglutide reversed nigrostriatal degeneration of rodent brain in rotenone-induced Parkinson’s disease. Inflammopharmacology 25:369–382
Baggio LL, Drucker DJ (2007) Biology of incretins: GLP-1 and GIP. Gastroenterology 132:2131–2157
Banchroft JD, Stevens A, Turner DR (1996) Theory and practice of histological techniques, 4th edn. Churchill Livingstone, London, pp 257–262
Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J, Kortesmaa J, Mercer A, Nielsen E, Ronnholm H, Wikstrom L (2008) Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J Neurosci Res 86:326–338
Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3:1301–1306
Betarbet R, Sherer TB and Greenamyre JT (2002) Animal models of Parkinson's disease. BioEssays 24:308–318
Bido S, Marti M, Morari M (2011) Amantadine attenuates levodopa-induced dyskinesia in mice and rats preventing the accompanying rise in nigral GABA levels. J Neurochem 118:1043–1055
Bisaglia M, Mammi S, Bubacco L (2007) Kinetic and structural analysis of the early oxidation products of dopamine: analysis of the interactions with alpha-synuclein. J Biol Chem 282:15597–15605
Bisaglia M, Tosatto L, Munari F, Tessari I, de Laureto PP, Mammi S, Bubacco L (2010) Dopamine quinones interact with alpha-synuclein to form unstructured adducts. Biochem Biophys Res Commun 394:424–428
Boyce S, Rupniak NM, Steventon MJ, Iversen SD (1990) Nigrostriatal damage is required for induction of dyskinesia by L-DOPA in squirrel monkeys. Clin Neuropharmacol 13:448–458
Cannon JR, Tapias V, Na HM, Honick AS, Drolet RE, Greenamyre JT (2009) A highly reproducible rotenone model of Parkinson’s disease. Neurobiol Dis 34:279–290
Carlsson A (2002) Treatment of Parkinson’s with L-DOPA. The early discovery phase, and a comment on current problems. J Neural Transm 109:777–787
Connolly BS, Lang AE (2014) Pharmacological treatment of Parkinson disease. JAMA 311:1670–1683
Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, Trapp S (2015) Distribution and characterisation of glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol Metab 4:718–731
Dexter DT, Jenner P (2013) Parkinson disease: from pathology to molecular disease mechanisms. Free Radic Biol Med 62:132–144
Di Monte DA, McCormack A, Petzinger G, Janson AM, Quik M, Langston WJ (2000) Relationship among nigrostriatal denervation, parkinsonism, and dyskinesia in the MPTP primate model. Mov Disord 15:459–466
Dodel RC, Berger K, Oertel WH (2001) Health-related quality of life and healthcareutilisation in patients with Parkinson’s disease: impact of motor fluctuations and dyskinesias. PharmacoEconomics 19:1013–1038
Dos-Santos-Pereira M, da-Silva CA, Guimaraes FS, Del-Bel E (2016) Co-administration of cannabidiol and capsazepine reduces L-DOPA-induced dyskinesia in mice: possible mechanism of action. Neurobiol Dis 94:179–195
Elsworth JD, Roth RH (1997) Dopamine synthesis, uptake, metabolism, and receptors: relevance to gene therapy of Parkinson’s disease. Exp Neurol 144:4–9
Fabbrini G, Brotchie JM, Grandas F, Nomoto M, Goetz CG (2007) Levodopa-induced dyskinesia. Mov Disord 22:1379–1389
Fahn S (2000) The spectrum of levodopa-induced dyskinesia. Ann Neurol 47:S2–S9 discussion S9–11
Gao HM, Hong JS, Zhang W, Liu B (2002) Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci 22:782–790
Gibb WR, Lees AJ (1988) A comparisons of clinical and pathological features of young and old-onset Parkinson’s disease. Neurology 38:1402–1406
Guridi J, Gonzalez-Redondo R, Obeso JA (2012) Clinical features, pathophysiology, and treatment of levodopa-induced dyskinesias in Parkinson’s disease. Parkinsons Dis 2012:943159
Haining RL, Achat-Mendes C (2017) Neuromelanin, one of the most overlooked molecules in modern medicine, is not a spectator. Neural Regen Res 12:372–375
Hamilton A, Patterson S, Porter D, Gault VA, Holscher C (2011) Novel GLP-1 mimetics developed to treat type 2 diabetes promote progenitor cell proliferation in the brain. J Neurosci Res 89:481–489
Harkavyi A, Whitton PS (2010) Glucagon-like peptide 1 receptor stimulation as a means of neuroprotection. Br J Pharmacol 159:495–501
Harkavyi A, Abuirmeileh A, Lever R, Kingsbury AE, Biggs CS, Whitton PS (2008) Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease. J Neuroinflammation 5:19
Hirsch EC, Hunot S, Damier P, Faucheux B (1998) Glial cells inflammation in Parkinson’s disease: a role in neurodegeneration? Ann Neurol 4:S115–S120
Hunter K, Holscher C (2012) Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci 13:33
ILAR (1996) Guide for the Care and Use of Laboratory Animals. National Academy Press, Washington
Jenner P (2003) The MPTP-treated primate as a model of motor complications in PD: primate model of motor complications. Neurology 61:S4–S11
Jenner P (2008) Molecular mechanisms of L-DOPA-induced dyskinesia. Nat Rev Neurosci 9:665–677
Kabel AM, Omar MS, Alhadhrami A, Alharthi SS, Alrobaian MM (2018) Linagliptin potentiates the effect of l-dopa on the behavioural, biochemical immunohistochemical changes in experimentally-induced parkinsonism: role of toll-like receptor 4, TGF-beta1, NF-kappaB glucagon-like peptide 1. Physiol Behav 188:108–118
Kandil EA, Sayed RH, Ahmed LA, Abd El Fattah MA, El-Sayeh BM (2018) Modulatory role of Nurr1 activation and thrombin inhibition in the neuroprotective effects of dabigatran etexilate in rotenone-induced Parkinson’s disease in rats. Mol Neurobiol 55:4078–4089
Kappe C, Tracy LM, Patrone C, Iverfeldt K, Sjoholm A (2012) GLP-1 secretion by microglial cells and decreased CNS expression in obesity. J Neuroinflammation 9:276
Kim S, Moon M, Park S (2009) Exendin-4 protects dopaminergic neurons by inhibition of microglial activation and matrix metalloproteinase-3 expression in an animal model of Parkinson’s disease. J Endocrinol 202:431–439
Knott C, Wilkin GP, Stern G (1999) Astrocytes and microglia in the substantia nigra and caudate-putamen in Parkinson’s disease. Parkinsonism Relat Disord 5:115–122
Kumar N, Van Gerpen JA, Bower JH, Ahlskog JE (2005) Levodopa-dyskinesia incidence by age of Parkinson’s disease onset. Mov Disord 20:342–344
Lane EL, Dunnett SB (2010) Pre-treatment with dopamine agonists influence L-dopa mediated rotations without affecting abnormal involuntary movements in the 6-OHDA lesioned rat. Behav Brain Res 213:66–72
Larsson M, Lietzau G, Nathanson D, Ostenson CG, Mallard C, Johansson ME, Nystrom T, Patrone C, Darsalia V (2016) Diabetes negatively affects cortical and striatal GABAergic neurons: an effect that is partially counteracted by exendin-4. Biosci Rep 36:e00421
Lee YS, Jun HS (2016) Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediat Inflamm 2016:3094642
Lee CS, Cenci MA, Schulzer M, Bjorklund A (2000) Embryonic ventral mesencephalic grafts improve levodopa-induced dyskinesia in a rat model of Parkinson’s disease. Brain 123:1365–1379
Lee CH, Yan B, Yoo KY, Choi JH, Kwon SH, Her S, Sohn Y, Hwang IK, Cho JH, Kim YM, Won MH (2011) Ischemia-induced changes in glucagon-like peptide-1 receptor and neuroprotective effect of its agonist, exendin-4, in experimental transient cerebral ischemia. J Neurosci Res 89:1103–1113
Lesser RP, Fahn S, Snider SR, Cote LJ, Isgreen WP, Barrett RE (1979) Analysis of the clinical problems in parkinsonism and the complications of long-term levodopa therapy. Neurology 29:1253–1260
Lew M (2007) Overview of Parkinson’s disease. Pharmacotherapy 27:155S–160S
Liu W, Jalewa J, Sharma M, Li G, Li L, Holscher C (2015) Neuroprotective effects of lixisenatide and liraglutide in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Neuroscience 303:42–50
Lohr KM, Bernstein AI, Stout KA, Dunn AR, Lazo CR, Alter SP, Wang M, Li Y, Fan X, Hess EJ, Yi H, Vecchio LM, Goldstein DS, Guillot TS, Salahpour A, Miller GW (2014) Increased vesicular monoamine transporter enhances dopamine release opposes Parkinson disease-related neurodegeneration in vivo. Proc Natl Acad Sci U S A 111:9977–9982
Lundblad M, Picconi B, Lindgren H, Cenci MA (2004) A model of L-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis 16:110–123
Lundblad M, Usiello A, Carta M, Hakansson K, Fisone G, Cenci MA (2005) Pharmacological validation of a mouse model of l-DOPA-induced dyskinesia. Exp Neurol 194:66–75
Mahmoud GA, El-Azab MF, Desoky AA, El-Sayed NM, Moustafa YM (2014) Activation of GLP-1R preserves dopaminergic neurons in rotenone-induced parkinsonism. In. 6th International Conference on Natural Toxins 27-B6. Avilable from: https://www.egynattox.com. Accessed 15-16 Dec 2014
Miller GW, Erickson JD, Perez JT, Penland SN, Mash DC, Rye DB, Levey AI (1999) Immunochemical analysis of vesicular monoamine transporter (VMAT2) protein in Parkinson’s disease. Exp Neurol 156:138–148
Nassar NN, Al-Shorbagy MY, Arab HH, Abdallah DM (2015) Saxagliptin: a novel antiparkinsonian approach. Neuropharmacology 89:308–317
Obeso JA, Rodriguez-Oroz MC, Chana P, Lera G, Rodriguez M, Olanow CW (2000) The evolution and origin of motor complications in Parkinson’s disease. Neurology 55:S13–S20 discussion S21–13
Olanow CW, Agid Y, Mizuno Y, Albanese A, Bonuccelli U, Damier P, De Yebenes J, Gershanik O, Guttman M, Grandas F, Hallett M, Hornykiewicz O, Jenner P, Katzenschlager R, Langston WJ, LeWitt P, Melamed E, Mena MA, Michel PP, Mytilineou C, Obeso JA, Poewe W, Quinn N, Raisman-Vozari R, Rajput AH, Rascol O, Sampaio C, Stocchi F (2004) Levodopa in the treatment of Parkinson’s disease: current controversies. Mov Disord 19:997–1005
Opacka-Juffry J, Ashworth S, Ahier RG, Hume SP (1998) Modulatory effects of L-DOPA on D2 dopamine receptors in rat striatum measured using in vivo microdialysis and PET. J Neural Transm 105:349–364
Pahwa R, Lyons KE (2009) Levodopa-related wearing-off in Parkinson’s disease: identification and management. Curr Med Res Opin 25:841–849
Paille V, Brachet P, Damier P (2004) Role of nigral lesion in the genesis of dyskinesias in a rat model of Parkinson’s disease. Neuroreport 15:561–564
Park S, Dong X, Fisher TL, Dunn S, Omer AK, Weir G, White MF (2006) Exendin-4 uses Irs2 signaling to mediate pancreatic beta cell growth and function. J Biol Chem 281:1159–1168
Parthsarathy V, Holscher C (2013) Chronic treatment with the GLP1 analogue liraglutide increases cell proliferation and differentiation into neurons in an AD mouse model. PLoS One 8:e58784
Perry T, Greig NH (2005) Enhancing central nervous system endogenous GLP-1 receptor pathways for intervention in Alzheimer’s disease. Curr Alzheimer Res 2:377–385
Quinn N, Critchley P, Marsden CD (1987) Young onset Parkinson’s disease. Mov Disord 2:73–91
Rajput AH, Fenton M, Birdi S, Macaulay R (1997) Is levodopa toxic to human substantia nigra? Mov Disord 12:634–638
Rascol O, Payoux P, Ory F, Ferreira JJ, Brefel-Courbon C, Montastruc JL (2003) Limitations of current Parkinson’s disease therapy. Ann Neurol 53(Suppl 3):S3–S12 discussion S12–15
Rivas E, de Ceballos ML, Nieto O, Fontenla JA (1999) In vivo effects of new inhibitors of catechol-O-methyl transferase. Br J Pharmacol 126:1667–1673
Schneider JS (1989) Levodopa-induced dyskinesia in parkinsonian monkeys: relationship to extent of nigrostriatal damage. Pharmacol Biochem Behav 34:193–196
Sharma JC, Macnamara L, Hasoon M, Vassallo M, Ross I (2006) Cascade of levodopa dose and weight-related dyskinesia in Parkinson’s disease (LD-WD-PD cascade). Parkinsonism Relat Disord 12:499–505
Sharma AN, Pise A, Sharma JN, Shukla P (2015) Glucagon-like peptide-1 (GLP-1) receptor agonist prevents development of tolerance to anti-anxiety effect of ethanol and withdrawal-induced anxiety in rats. Metab Brain Dis 30:719–730
Sulzer D, Zecca L (2000) Intraneuronal dopamine-quinone synthesis: a review. Neurotox Res 1:181–195
Sulzer D, Bogulavsky J, Larsen KE, Behr G, Karatekin E, Kleinman MH, Turro N, Krantz D, Edwards RH, Greene LA, Zecca L (2000) Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc Natl Acad Sci U S A 97:11869–11874
Taylor TN, Alter SP, Wang M, Goldstein DS, Miller GW (2014) Reduced vesicular storage of catecholamines causes progressive degeneration in the locus ceruleus. Neuropharmacology 76(Pt A):97–105
Teema AM, Zaitone SA, Moustafa YM (2016) Ibuprofen or piroxicam protects nigral neurons and delays the development of l-dopa induced dyskinesia in rats with experimental parkinsonism: influence on angiogenesis. Neuropharmacology 107:432–450
Teismann P, Tieu K, Cohen O, Choi DK, Wu DC, Marks D, Vila M, Jackson-Lewis V, Przedborski S (2003) Pathogenic role of glial cells in Parkinson’s disease. Mov Disord 18:121–129
Toulouse A, Sullivan AM (2008) Progress in Parkinson’s disease—where do we stand? Prog Neurobiol 85:376–392
von Wrangel C, Schwabe K, John N, Krauss JK, Alam M (2015) The rotenone-induced rat model of Parkinson’s disease: behavioral and electrophysiological findings. Behav Brain Res 279:52–61
Wang Q, Liu Y, Zhou J (2015) Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl Neurodegener 4:19
Yulug B, Hanoglu L, Kilic E (2015) The neuroprotective role of vesicular monoamine transporter 2 in neurodegenerative diseases. Med Chem 11:104–108
Zaitone SA, Abo-Elmatty DM, Elshazly SM (2012a) Piracetam, vinpocetine ameliorate rotenone-induced parkinsonism in rats. Indian J Pharmacol 44:774–779
Zaitone SA, Abo-Elmatty DM, Shaalan AA (2012b) Acetyl-L-carnitine alpha-lipoic acid affect rotenone-induced damage in nigral dopaminergic neurons of rat brain, implication for Parkinson’s disease therapy. Pharmacol Biochem Behav 100:347–360
Zaitone SA, Hammad LN, Farag NE (2013) Antioxidant potential of melatonin enhances the response to L-dopa in 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine-parkinsonian mice. Pharmacol Rep 65:1213–1226
Zecca L, Wilms H, Geick S, Claasen JH, Brandenburg LO, Holzknecht C, Panizza ML, Zucca FA, Deuschl G, Sievers J, Lucius R (2008) Human neuromelanin induces neuroinflammation and neurodegeneration in the rat substantia nigra: implications for Parkinson’s disease. Acta Neuropathol 116:47–55
Zhang W, Phillips K, Wielgus AR, Liu J, Albertini A, Zucca FA, Faust R, Qian SY, Miller DS, Chignell CF, Wilson B, Jackson-Lewis V, Przedborski S, Joset D, Loike J, Hong JS, Sulzer D, Zecca L (2011) Neuromelanin activates microglia and induces degeneration of dopaminergic neurons: implications for progression of Parkinson’s disease. Neurotox Res 19:63–72
Zhang W, Zecca L, Wilson B, Ren HW, Wang YJ, Wang XM, Hong JS (2013) Human neuromelanin: an endogenous microglial activator for dopaminergic neuron death. Front Biosci 5:1–11
Zucca FA, Basso E, Cupaioli FA, Ferrari E, Sulzer D, Casella L, Zecca L (2014) Neuromelanin of the human substantia nigra: an update. Neurotox Res 25:13–23
Acknowledgments
The authors thank Alfacure Company (Bader City, Egypt) for kindly providing L-dopa and carbidopa powder.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Badawi, G.A., Abd El Fattah, M.A., Zaki, H.F. et al. Sitagliptin and Liraglutide Modulate L-dopa Effect and Attenuate Dyskinetic Movements in Rotenone-Lesioned Rats. Neurotox Res 35, 635–653 (2019). https://doi.org/10.1007/s12640-019-9998-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-019-9998-3