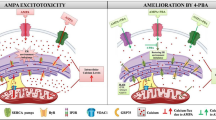
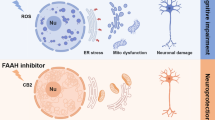
Abstract
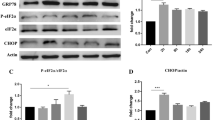
Excessive stimulation of ionotropic glutamate receptor is associated with glutamate-mediated excitotoxicity, thereby causing oxidative imbalance and mitochondrial dysfunction, resulting in the excitotoxic death of neurons. Eminent role of endoplasmic reticulum under glutamate-induced excitotoxicity has been highlighted in numerous literatures which have been observed to trigger endoplasmic reticulum stress (ER stress) as well as regulating oxidative stress. However, combating ER stress in excitotoxic neurons can provide a novel approach to alleviate the mitochondrial dysfunctioning and ROS generation. Therefore, we propose to investigate the cross-communication of α-amino-3-hydroxy-5-methyl-4-isoxzole-propionate (AMPA) excitotoxicity-induced oxidative injury with ER stress by employing ER stress inhibitor—4-phenlybutyric acid (4-PBA). Male SD rats were divided into four groups viz sham group (group 1), AMPA (10 mM)-induced excitotoxic group (group 2), curative group of AMPA-induced excitotoxic animals given 4-PBA at a dose of 100 mg/kg body weight (group 3), and alone 4-PBA treatment group (100 mg/kg body weight) (group 4). Animals were sacrificed after 15 days of treatment, and hippocampi were analyzed for histopathological examination, ROS, inflammatory markers, mitochondrial dysfunction, and ER stress markers. AMPA-induced excitotoxicity exhibited a significant increase in the levels of ROS, upregulated ER stress markers, inflammation markers, and compromised mitochondrial functioning in the hippocampus. However, 4-PBA administration significantly curtailed the AMPA-induced excitotoxic insult. This study suggests that targeting ER stress with a chemical chaperone can provide a better therapeutic intervention for neurological disorders involving excitotoxicity, and thus, it opens a new avenue to screen chemical chaperones for the therapeutic modalities.





Similar content being viewed by others
References
Bernal F, Petegnief V, Rodríguez MJ, Ursu G, Pugliese M, Mahy N (2009) Nimodipine inhibits TMB-8 potentiation of AMPA-induced hippocampal neurodegeneration. J Neurosci Res 87:1240–1249
Best TM, Fiebig R, Corr DT, Brickson S, Ji L (1999) Free radical activity, antioxidant enzyme, and glutathione changes with muscle stretch injury in rabbits. J Appl Physiol 87:74–82
Bhandary B, Marahatta A, Kim HR, Chae HJ (2013) An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int J Mol Sci 14:434–456
Cao J, Wang Z, Mi W, Zuo Z (2014) Isoflurane unveils a critical role of glutamate transporter type 3 in regulating hippocampal GluR1 trafficking and context-related learning and memory in mice. Neuroscience 11:58–64
Chen C, Li B, Cheng G, Yang X, Zhao N, Shi R (2018) Amentoflavone ameliorates Aβ1-42-induced memory deficits and oxidative stress in cellular and rat model. Neurochem Res 43:857–868
Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW, Chuang YC (2011) Roles of oxidative stress, apoptosis, PGC-1α and mitochondrial biogenesis in cerebral ischemia. Int J Mol Sci 12:7199–7215
Chuang Y-C, Chang AYW, Lin J-W, Hsu SP, Chan SH (2004) Mitochondrial dysfunction and ultrastructural damage in the hippocampus during kainic acid-induced status epilepticus in the rat. Epilepsia 45:1202–1209
Concannon CG, Ward MW, Bonner HP, Kuroki K, Tuffy LP, Bonner CT, Woods I, Engel T, Henshall DC, Prehn JH (2008) NMDA receptor-mediated excitotoxic neuronal apoptosis in vitro and in vivo occurs in an ER stress and PUMA independent manner. J Neurochem 105:891–903
Dhanda S, Sunkaria A, Halder A, Sandhir R (2018) Mitochondrial dysfunctions contribute to energy deficits in rodent model of hepatic encephalopathy. Metab Brain Dis 33:209–223
Dong XX, Wang Y, Qin ZH (2009) Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin Acta Pharmacol Sin 30:379–387
Dong Y, Kalueff AV, Song C (2017) N-methyl-d-aspartate receptor-mediated calcium overload and endoplasmic reticulum stress are involved in interleukin-1beta-induced neuronal apoptosis in rat hippocampus. J Neuroimmunol 307:7–13
Emerit J, Edeas M, Bricaire F (2004) Neurodegenerative diseases and oxidative stress. Biomed Pharmacother 58:39–46
Hartl FU, Bracher A, Hayer-Hartl M (2011) Molecular chaperones in protein folding and proteostasis. Nature 475:324–332
Hilton GD, Nunez JL, Bambrick L, Thompson SM, McCarthy MM (2006) Glutamate-mediated excitotoxicity in neonatal hippocampal neurons is mediated by mGluR-induced release of Ca++ from intracellular stores and is prevented by estradiol. Eur J Neurosci 24:3008–3016
Isaac JTR, Ashby MC, McBain CJ (2007) The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 54:859–871
Jung KH, Chu K, Lee ST, Park HK, Kim JH, Kang KM, Kim M, Lee SKRJ (2009) Augmentation of nitrite therapy in cerebral ischemia by NMDA receptor inhibition. Biochem Biophys Res Commun 378:507–512
Kaufman RJ, Malhotra JD (2014) Calcium trafficking integrates endoplasmic reticulum function with mitochondrial bioenergetics. Biochim Biophys Acta 1843:2233–2239
Kavirajan H, Schneider LS (2007) Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: a meta-analysis of randomised controlled trials. Lancet Neurol 6:782–792
King TE, Howard RL (1967) [52] Preparations and properties of soluble NADH dehydrogenases from cardiac muscle. Methods Enzymol 10:275–294
King TE, Ohnishi T, Winter DB, Wu JT (1976) Biochemical and EPR probes for structure-function studies of iron sulfur centers of succinate dehydrogenase. Adv Exp Med Biol 74:182–227
Kovacic P, Somanathan R (2010) Clinical physiology and mechanism of dizocilpine (MK-801): electron transfer, radicals, redox metabolites and bioactivity. Oxidative Med Cell Longev 3:13–22
Liu W, Liu R, Chun JT, Hoe W, Schreiber SS, Baudry M (2001) Kainate excitotoxicity in organotypic hippocampal slice cultures: evidence for multiple apoptotic pathways. Brain Res 916:239–248
Ndountse LT, Chan HM (2009) Role of N-methyl-D-aspartate receptors in polychlorinated biphenyl mediated neurotoxicity. Toxicol Lett 184:50–55
Nicholls DG (2004) Mitochondrial dysfunction and glutamate excitotoxicity studied in primary neuronal cultures. Curr Mol Med 4:149–177
Paschen W, Mengesdorf T (2005) Endoplasmic reticulum stress response and neurodegeneration. Cell Calcium 38:409–415
Pereira EPL, Braga-De-Souza S, Santos CC, Amparo J, Short Ferreira R, Nuñez-Figueredo Y, Gonzaga Fernandez L, Ribeiro PR, Braga-de-Souza S, Amaral da Silva VD, Lima Costa S (2017) Amburana cearensis seed extract protects brain mitochondria from oxidative stress and cerebellar cells from excitotoxicity induced by glutamate. Rev Bras 27:199–205
Petegnief V, Saura J, Dewar D, Cummins DJ, Dragunow M, Mahy N (1999) Long-term effects of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate and 6-nitro-7-sulphamoylbenzo(f)quinoxaline-2,3-dione in the rat basal ganglia: calcification, changes in glutamate receptors and glial reactions. Neuroscience 94:105–115
Petit JM, Maftah A, Ratinaud MH, Julien R (1992) 10N-nonyl acridine orange interacts with cardiolipin and allows the quantification of this phospholipid in isolated mitochondria. Eur J Biochem 209:267–273
Prentice H, Modi JP, Wu JY (2015) Mechanisms of neuronal protection against excitotoxicity, endoplasmic reticulum stress, and mitochondrial dysfunction in stroke and neurodegenerative diseases. Oxid Med Cell Longev 2015:7 pages
Racay P, Tatarkova Z, Chomova M, Hatok J, Kaplan P, Dobrota D (2009) Mitochondrial calcium transport and mitochondrial dysfunction after global brain ischemia in rat hippocampus. Neurochem Res 34:1469–1478
Rego AC, Oliveira CR (2003) Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochem Res 28:1563–1574
Ruiz A, Matute C, Alberdi E (2010) Intracellular Ca2+ release through ryanodine receptors contributes to AMPA receptor-mediated mitochondrial dysfunction and ER stress in oligodendrocytes. Cell Death Dis 1:e54
Salminen A, Kauppinen A, Suuronen T, Kaarniranta K, Ojala J (2009) ER stress in Alzheimer’s disease: a novel neuronal trigger for inflammation and Alzheimer’s pathology. J Neuroinflammation 6:1–13
Sharma S, Verma S, Kapoor M, Saini A, Nehru B (2016) Alzheimer’s disease like pathology induced six weeks after aggregated amyloid-beta injection in rats: increased oxidative stress and impaired long-term memory with anxiety-like behavior. Neurol Res 38:838–850
Sokka AL, Putkonen N, Mudo G, Pryazhnikov E, Reijonen S, Khiroug L, Belluardo N, Lindholm D, Korhonen L (2007) Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci 27:901–908
Sottocasa GL, Kuylenstierna B, Ernster L, Bergstrand A (1967) An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol 32:415–438
Srinivasan K, Sharma SS (2011) Sodium phenylbutyrate ameliorates focal cerebral ischemic/reperfusion injury associated with comorbid type 2 diabetes by reducing endoplasmic reticulum stress and DNA fragmentation. Behav Brain Res 225:110–116
Van Den Bosch L, Van Damme P, Bogaert E, Robberecht W (2006) The role of excitotoxicity in the pathogenesis of amyotrophic lateral sclerosis. Biochim Biophys Acta Mol basis Dis 1762:1068–1082
Varbiro G, Veres B, Sumegi B (2001) Direct effect of Taxol on free radical formation and mitochondrial permeability transition. Free Radic Biol Med 31:548–558
Vezzani A, Granata T (2005) Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia 46:1724–1743
Wiley JC, Pettan-Brewer C, Ladiges WC (2011) Phenylbutyric acid reduces amyloid plaques and rescues cognitive behavior in AD transgenic mice. Aging Cell 10:418–428
Wu J, Gao L, Shang L, Wang G, Wei N, Chu T, Chen S, Zhang Y, Huang J, Wang J, Lin R (2017) Ecdysterones from Rhaponticum carthamoides (Willd.) Iljin reduce hippocampal excitotoxic cell loss and upregulate mTOR signaling in rats. Fitoterapia 119:158–167
Zeeshan HMA, Lee GH, Kim HR, Chae HJ (2016) Endoplasmic reticulum stress and associated ROS. Int J Mol Sci 17:327
Funding Source
This work was supported by the University Grant Commission-Basic Scientific Research (UGC-BSR) (F.25-1/2013-14(BSR)/7-209/2009(BSR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animals were maintained as per the principles and guidelines of the Ethics Committee of the Animal Care of Panjab University in accordance with the Indian national law on animal care and use. The animal experimental protocols were approved by Institutional Animal Ethics Committee (reference no. PU/IAEC/S/15/42) and conducted according to the Indian National Science Academy Guidelines for the use and care of experimental animals.
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
ESM 1
(PNG 1437 kb)
Rights and permissions
About this article
Cite this article
Bhardwaj, A., Bhardwaj, R., Dhawan, D.K. et al. Exploring the Effect of Endoplasmic Reticulum Stress Inhibition by 4-Phenylbutyric Acid on AMPA-Induced Hippocampal Excitotoxicity in Rat Brain. Neurotox Res 35, 83–91 (2019). https://doi.org/10.1007/s12640-018-9932-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-018-9932-0