Abstract
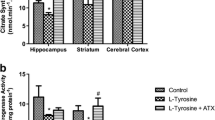
Tyrosinemia type II is an inborn error of metabolism caused by a deficiency in the activity of the enzyme tyrosine aminotransferase, leading to tyrosine accumulation in the body. Although the mechanisms involved are still poorly understood, several studies have showed that higher levels of tyrosine are related to oxidative stress and therefore may affect the cholinergic system. Thus, the aim of this study was to investigate the effects of chronic administration of L-tyrosine on choline acetyltransferase activity (ChAT) and acetylcholinesterase (AChE) in the brain of rats. Moreover, we also examined the effects of one antioxidant treatment (N-acetylcysteine (NAC) + deferoxamine (DFX)) on cholinergic system. Our results showed that the chronic administration of L-tyrosine decreases the ChAT activity in the cerebral cortex, while the AChE activity was increased in the hippocampus, striatum, and cerebral cortex. Moreover, we found that the antioxidant treatment was able to prevent the decrease in the ChAT activity in the cerebral cortex. However, the increase in AChE activity induced by L-tyrosine was partially prevented the in the hippocampus and striatum, but not in the cerebral cortex. Our results also showed no differences in the aversive and spatial memory after chronic administration of L-tyrosine. In conclusion, the results of this study demonstrated an increase in AChE activity in the hippocampus, striatum, and cerebral cortex and an increase of ChAT in the cerebral cortex, without cognitive impairment. Furthermore, the alterations in the cholinergic system were partially prevented by the co-administration of NAC and DFX. Thus, the restored central cholinergic system by antioxidant treatment further supports the view that oxidative stress may be involved in the pathophysiology of tyrosinemia type II.





Similar content being viewed by others
References
Aitken RJ, Harkiss D, Knox W, Paterson M, Irvine DS (1998) A novel signal transduction cascade in capacitating human spermatozoa characterised by a redox-regulated, cAMP-mediated induction of tyrosine phosphorylation. J Cell Sci 111(Pt 5):645–656
Arent CO, Reus GZ, Abelaira HM, Ribeiro KF, Steckert AV, Mina F, Dal-Pizzol F, Quevedo J (2012) Synergist effects of n-acetylcysteine and deferoxamine treatment on behavioral and oxidative parameters induced by chronic mild stress in rats. Neurochem Int 61:1072–1080
Aruoma OI, Halliwell B, Hoey BM, Butler J (1989) The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med 6:593–597
Auld DS, Kornecook TJ, Bastianetto S, Quirion R (2002) Alzheimer’s disease and the basal forebrain cholinergic system: relations to beta-amyloid peptides, cognition, and treatment strategies. Prog Neurobiol 68:209–245
Balansky R, Izzotti A, Scatolini L, D'Agostini F, De Flora S (1996) Induction by carcinogens and chemoprevention by N-acetylcysteine of adducts to mitochondrial DNA in rat organs. Cancer Res 56:1642–1647
Ballard CG, Greig NH, Guillozet-Bongaarts AL, Enz A, Darvesh S (2005) Cholinesterases: roles in the brain during health and disease. Curr Alzheimer Res 2:307–318
Baratz-Goldstein R, Deselms H, Heim LR, Khomski L, Hoffer BJ, Atlas D, Pick CG (2016) Thioredoxin-mimetic-peptides protect cognitive function after mild traumatic brain injury (mTBI). PLoS One 11:e0157064
Barichello T, Machado RA, Constantino L, Valvassori SS, Reus GZ, Martins MR, Petronilho F, Ritter C, Quevedo J, Dal-Pizzol F (2007) Antioxidant treatment prevented late memory impairment in an animal model of sepsis. Crit Care Med 35:2186–2190
Barichello T, Martins MR, Reinke A, Feier G, Ritter C, Quevedo J, Dal-Pizzol F (2005) Cognitive impairment in sepsis survivors from cecal ligation and perforation. Crit Care Med 33:221–223 discussion 262-223
Bartus RT, Dean RL 3rd, Beer B, Lippa AS (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217:408–414
Bavarsad Shahripour R, Harrigan MR, Alexandrov AV (2014) N-acetylcysteine (NAC) in neurological disorders: mechanisms of action and therapeutic opportunities. Brain Behav 4:108–122
Belen Herrero M, Chatterjee S, Lefievre L, de Lamirande E, Gagnon C (2000) Nitric oxide interacts with the cAMP pathway to modulate capacitation of human spermatozoa. Free Radic Biol Med 29:522–536
Benrahmoune M, Therond P, Abedinzadeh Z (2000) The reaction of superoxide radical with N-acetylcysteine. Free Radic Biol Med 29:775–782
Bevilaqua LR, Kerr DS, Medina JH, Izquierdo I, Cammarota M (2003) Inhibition of hippocampal Jun N-terminal kinase enhances short-term memory but blocks long-term memory formation and retrieval of an inhibitory avoidance task. Eur J Neurosci 17:897–902
Blokland A (1995) Acetylcholine: a neurotransmitter for learning and memory? Brain Res Brain Res Rev 21:285–300
Bohnen NI, Albin RL (2011) The cholinergic system and Parkinson disease. Behav Brain Res 221:564–573
Bonefeld BE, Elfving B, Wegener G (2008) Reference genes for normalization: a study of rat brain tissue. Synapse 62:302–309
Bongiovanni R, Yamamoto BK, Simpson C, Jaskiw GE (2003) Pharmacokinetics of systemically administered tyrosine: a comparison of serum, brain tissue and in vivo microdialysate levels in the rat. J Neurochem 87:310–317
Bruce G, Hersh LB (1989) The phosphorylation of choline acetyltransferase. Neurochem Res 14:613–620
Bustin SA, Benes V, Garson J, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley G, Wittwer CT, Schjerling P, Day PJ, Abreu M, Aguado B, Beaulieu JF, Beckers A, Bogaert S, Browne JA, Carrasco-Ramiro F, Ceelen L, Ciborowski K, Cornillie P, Coulon S, Cuypers A, De Brouwer S, De Ceuninck L, De Craene J, De Naeyer H, De Spiegelaere W, Deckers K, Dheedene A, Durinck K, Ferreira-Teixeira M, Fieuw A, Gallup JM, Gonzalo-Flores S, Goossens K, Heindryckx F, Herring E, Hoenicka H, Icardi L, Jaggi R, Javad F, Karampelias M, Kibenge F, Kibenge M, Kumps C, Lambertz I, Lammens T, Markey A, Messiaen P, Mets E, Morais S, Mudarra-Rubio A, Nakiwala J, Nelis H, Olsvik PA, Perez-Novo C, Plusquin M, Remans T, Rihani A, Rodrigues-Santos P, Rondou P, Sanders R, Schmidt-Bleek K, Skovgaard K, Smeets K, Tabera L, Toegel S, Van Acker T, Van den Broeck W, Van der Meulen J, Van Gele M, Van Peer G, Van Poucke M, Van Roy N, Vergult S, Wauman J, Tshuikina-Wiklander M, Willems E, Zaccara S, Zeka F, Vandesompele J (2013) The need for transparency and good practices in the qPCR literature. Nat Methods 10:1063–1067
Carvalho-Silva M, Gomes LM, Scaini G, Rebelo J, Damiani AP, Pereira M, Andrade VM, Gava FF, Valvassori SS, Schuck PF, Ferreira GC, Streck EL (2017) Omega-3 fatty acid supplementation decreases DNA damage in brain of rats subjected to a chemically induced chronic model of Tyrosinemia type II. Metab Brain Dis 32:1043–1050
Cassol OJ Jr, Rezin GT, Petronilho FC, Scaini G, Goncalves CL, Ferreira GK, Roesler R, Schwartsmann G, Dal-Pizzol F, Streck EL (2010) Effects of N-acetylcysteine/deferoxamine, taurine and RC-3095 on respiratory chain complexes and creatine kinase activities in rat brain after sepsis. Neurochem Res 35:515–521
Chao LP, Wolfgram F (1973) Purification and some properties of choline acetyltransferase (EC 2.3.1.6) from bovine brain. J Neurochem 20:1075–1081
Chuang DT, Shih VE (2001) Maple syrup urine disease (branched-chain ketoaciduria). In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 1971–2005
Cocco T, Sgobbo P, Clemente M, Lopriore B, Grattagliano I, Di Paola M, Villani G (2005) Tissue-specific changes of mitochondrial functions in aged rats: effect of a long-term dietary treatment with N-acetylcysteine. Free Radic Biol Med 38:796–805
Contestabile A, Ciani E, Contestabile A (2008) The place of choline acetyltransferase activity measurement in the “cholinergic hypothesis” of neurodegenerative diseases. Neurochem Res 33:318–327
Costa M, Bernardi J, Fiuza T, Costa L, Brandao R, Pereira ME (2016) N-acetylcysteine protects memory decline induced by streptozotocin in mice. Chem Biol Interact 253:10–17
Cuzzocrea S, Mazzon E, Costantino G, Serraino I, De Sarro A, Caputi AP (2000a) Effects of n-acetylcysteine in a rat model of ischemia and reperfusion injury. Cardiovasc Res 47:537–548
Cuzzocrea S, Mazzon E, Costantino G, Serraino I, Dugo L, Calabro G, Cucinotta G, De Sarro A, Caputi AP (2000b) Beneficial effects of n-acetylcysteine on ischaemic brain injury. Br J Pharmacol 130:1219–1226
Davies P, Maloney AJ (1976) Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 2:1403
de Andrade RB, Gemelli T, Rojas DB, Bonorino NF, Costa BM, Funchal C, Dutra-Filho CS, Wannmacher CM (2015) Creatine and pyruvate prevent the alterations caused by tyrosine on parameters of oxidative stress and enzyme activities of phosphoryltransfer network in cerebral cortex of Wistar rats. Mol Neurobiol 51:1184–1194
De Pra SD, Ferreira GK, Carvalho-Silva M, Vieira JS, Scaini G, Leffa DD, Fagundes GE, Bristot BN, Borges GD, Ferreira GC, Schuck PF, Andrade VM, Streck EL (2014) L-tyrosine induces DNA damage in brain and blood of rats. Neurochem Res 39:202–207
De Vries N, De Flora S (1993) N-acetyl-l-cysteine. J Cell Biochem Suppl 17F:270–277
Di-Pietro PB, Dias ML, Scaini G, Burigo M, Constantino L, Machado RA, Dal-Pizzol F, Streck EL (2008) Inhibition of brain creatine kinase activity after renal ischemia is attenuated by N-acetylcysteine and deferoxamine administration. Neurosci Lett 434:139–143
Dobransky T, Davis WL, Xiao GH, Rylett RJ (2000) Expression, purification and characterization of recombinant human choline acetyltransferase: phosphorylation of the enzyme regulates catalytic activity. Biochemical J 349:141–151
Dodd S, Dean O, Copolov DL, Malhi GS, Berk M (2008) N-acetylcysteine for antioxidant therapy: pharmacology and clinical utility. Expert Opin Biol Ther 8:1955–1962
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, Butterfield DA, Morley JE (2003) The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem 84:1173–1183
Ferreira GK, Carvalho-Silva M, Gomes LM, Scaini G, Teixeira LJ, Mota IT, Schuck PF, Ferreira GC, Streck EL (2015) The characterization of neuroenergetic effects of chronic L-tyrosine administration in young rats: evidence for striatal susceptibility. Metab Brain Dis 30:215–221
Ferreira GK, Carvalho-Silva M, Goncalves CL, Vieira JS, Scaini G, Ghedim FV, Deroza PF, Zugno AI, Pereira TC, Oliveira GM, Kist LW, Bogo MR, Schuck PF, Ferreira GC, Streck EL (2012) L-tyrosine administration increases acetylcholinesterase activity in rats. Neurochem Int 61:1370–1374
Ferreira GK, Jeremias IC, Scaini G, Carvalho-Silva M, Gomes LM, Furlanetto CB, Morais MO, Schuck PF, Ferreira GC, Streck EL (2013a) Effect of acute and chronic administration of L-tyrosine on nerve growth factor levels in rat brain. Neurochem Res 38:1742–1746
Ferreira GK, Scaini G, Carvalho-Silva M, Gomes LM, Borges LS, Vieira JS, Constantino LS, Ferreira GC, Schuck PF, Streck EL (2013b) Effect of L-tyrosine in vitro and in vivo on energy metabolism parameters in brain and liver of young rats. Neurotox Res 23:327–335
Ferreira GK, Scaini G, Jeremias IC, Carvalho-Silva M, Goncalves CL, Pereira TC, Oliveira GM, Kist LW, Bogo MR, Schuck PF, Ferreira GC, Streck EL (2014) An evaluation of the effects of acute and chronic L-tyrosine administration on BDNF levels and BDNF mRNA expression in the rat brain. Mol Neurobiol 49:734–740
Fine A, Hoyle C, Maclean CJ, Levatte TL, Baker HF, Ridley RM (1997) Learning impairments following injection of a selective cholinergic immunotoxin, ME20.4 IgG-saporin, into the basal nucleus of Meynert in monkeys. Neuroscience 81:331–343
Flora SJ (2009) Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxidative Med Cell Longev 2:191–206
Fodale V, Quattrone D, Trecroci C, Caminiti V, Santamaria LB (2006) Alzheimer’s disease and anaesthesia: implications for the central cholinergic system. Br J Anaesth 97:445–452
Fu AL, Dong ZH, Sun MJ (2006) Protective effect of N-acetyl-L-cysteine on amyloid beta-peptide-induced learning and memory deficits in mice. Brain Res 1109:201–206
Gold PE (2003a) Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol Learn Mem 80:194–210
Gold PE (2003b) Acetylcholine: cognitive and brain functions. Neurobiol Learn Mem 80:177
Goldsmith LA (1978) Molecular biology and molecular pathology of a newly described molecular disease—tyrosinemia II (the Richner-Hanhart syndrome). Exp Cell Biol 46:96–113
Grifman M, Arbel A, Ginzberg D, Glick D, Elgavish S, Shaanan B, Soreq H (1997) In vitro phosphorylation of acetylcholinesterase at non-consensus protein kinase a sites enhances the rate of acetylcholine hydrolysis. Brain Res Mol Brain Res 51:179–187
Hata F, Takeyasu K, Morikawa Y, Lai RT, Ishida H, Yoshida H (1980) Specific changes in the cholinergic system in guinea-pig vas deferens after denervation. J Pharmacol Exp Ther 215:716–722
Held PK (2006) Disorders of tyrosine catabolism. Mol Genet Metab 88:103–106
Hicdonmez T, Kanter M, Tiryaki M, Parsak T, Cobanoglu S (2006) Neuroprotective effects of N-acetylcysteine on experimental closed head trauma in rats. Neurochem Res 31:473–481
Hyde TM, Crook JM (2001) Cholinergic systems and schizophrenia: primary pathology or epiphenomena? J Chem Neuroanat 22:53–63
Izquierdo I (1998) Involvement of hippocampal NMDA receptors in retention of shuttle avoidance conditioning in rats. Neurobiol Learn Mem 31:1601–1604
Kapczinski F (2008) Effect of N-acetylcysteine and/or deferoxamine on oxidative stress and hyperactivity in an animal model of mania. Br J Psychiatry J Ment Sci 32:1064–1068
Khan M, Sekhon B, Jatana M, Giri S, Gilg AG, Sekhon C, Singh I, Singh AK (2004) Administration of N-acetylcysteine after focal cerebral ischemia protects brain and reduces inflammation in a rat model of experimental stroke. J Neurosci Res 76:519–527
Kuznetsova E, Schliebs R (2013) Beta-amyloid, cholinergic transmission, and cerebrovascular system—a developmental study in a mouse model of Alzheimer’s disease. Curr Pharm Des 19:6749–6765
Lackner P, Beer R, Heussler V, Goebel G, Rudzki D, Helbok R, Tannich E, Schmutzhard E (2006) Behavioural and histopathological alterations in mice with cerebral malaria. Neuropathol Appl Neurobiol 32:177–188
Leclerc P, de Lamirande E, Gagnon C (1997) Regulation of protein-tyrosine phosphorylation and human sperm capacitation by reactive oxygen derivatives. Free Radic Biol Med 22:643–656
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lustig C, Sarter M (2016) Attention and the cholinergic system: relevance to schizophrenia. Curr Top Behav Neurosci 28:327–362
Macedo LG, Carvalho-Silva M, Ferreira GK, Vieira JS, Olegario N, Goncalves RC, Vuolo FS, Ferreira GC, Schuck PF, Dal-Pizzol F, Streck EL (2013) Effect of acute administration of L-tyrosine on oxidative stress parameters in brain of young rats. Neurochem Res 38:2625–2630
Macsai MS, Schwartz TL, Hinkle D, Hummel MB, Mulhern MG, Rootman D (2001) Tyrosinemia type II: nine cases of ocular signs and symptoms. Am J Ophthalmol 132:522–527
Majocha R, Baldessarini RJ (1984) Tolerance to an anticholinergic agent is paralleled by increased binding to muscarinic receptors in rat brain and increased behavioral response to a centrally active cholinomimetic. Life Sci 35:2247–2255
Manyam BV, Giacobini E, Colliver JA (1990) Cerebrospinal fluid acetylcholinesterase and choline measurements in Huntington’s disease. J Neurol 237:281–284
Martinez M, Hernandez AI, Martinez N (2000) N-acetylcysteine delays age-associated memory impairment in mice: role in synaptic mitochondria. Brain Res 855:100–106
Mitchell GA, Grompe M, Lambert M, Tanguay RM (2001) Hypertyrosinemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, vol 8. Mc Graw-Hill, New York, pp 1977–1982
Mitchell GA, Grompe M, Lambert M, Tanguay RM (2013) Hypertyrosinemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease. Mc Graw-Hill, New York
Morre MC, Hefti F, Wurtman RJ (1980) Regional tyrosine levels in rat brain after tyrosine administration. J Neural Transm 49:45–50
Mufson EJ, Counts SE, Perez SE, Ginsberg SD (2008) Cholinergic system during the progression of Alzheimer’s disease: therapeutic implications. Expert Rev Neurother 8:1703–1718
Oda Y (1999) Choline acetyltransferase: the structure, distribution and pathologic changes in the central nervous system. Pathol Int 49:921–937
Pan J, Xiao Q, Sheng CY, Hong Z, Yang HQ, Wang G, Ding JQ, Chen SD (2009) Blockade of the translocation and activation of c-Jun N-terminal kinase 3 (JNK3) attenuates dopaminergic neuronal damage in mouse model of Parkinson’s disease. Neurochem Int 54:418–425
Pepeu G (1972) Cholinergic neurotransmission in the central nervous system. Arch Int Pharmacodyn Ther 196(Suppl 196):229
Pinho RA, Silveira PC, Silva LA, Luiz Streck E, Dal-Pizzol F, Moreira JC (2005) N-acetylcysteine and deferoxamine reduce pulmonary oxidative stress and inflammation in rats after coal dust exposure. Environ Res 99:355–360
Quevedo J (2014) Evaluation of acetylcholinesterase activity and behavioural alterations induced by ketamine in an animal model of schizophrenia. Exp Biol Med (Maywood, NJ) 26:43–50
Quevedo J, Vianna M, Zanatta MS, Roesler R, Izquierdo I, Jerusalinsky D, Quillfeldt JA (1997) Involvement of mechanisms dependent on NMDA receptors, nitric oxide and protein kinase A in the hippocampus but not in the caudate nucleus in memory. Behav Pharmacol 8:713–717
Quevedo J, Vianna MR, Roesler R, de Paris F, Izquierdo I, Rose SP (1999) Two time windows of anisomycin-induced amnesia for inhibitory avoidance training in rats: protection from amnesia by pretraining but not pre-exposure to the task apparatus. Learn Mem 6:600–607
Ramos AC, Ferreira GK, Carvalho-Silva M, Furlanetto CB, Goncalves CL, Ferreira GC, Schuck PF, Streck EL (2013) Acute administration of l-tyrosine alters energetic metabolism of hippocampus and striatum of infant rats. Int J Dev Neurosci 31:303–307
Ritter C, Andrades ME, Reinke A, Menna-Barreto S, Moreira JC, Dal-Pizzol F (2004) Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit Care Med 32:342–349
Ritter C, da Cunha AA, Echer IC, Andrades M, Reinke A, Lucchiari N, Rocha J, Streck EL, Menna-Barreto S, Moreira JC, Dal-Pizzol F (2006) Effects of N-acetylcysteine plus deferoxamine in lipopolysaccharide-induced acute lung injury in the rat. Crit Care Med 34:471–477
Rodrigues FS, Souza MA, Magni DV, Ferreira AP, Mota BC, Cardoso AM, Paim M, Xavier LL, Ferreira J, Schetinger MR, Da Costa JC, Royes LF, Fighera MR (2013) N-acetylcysteine prevents spatial memory impairment induced by chronic early postnatal glutaric acid and lipopolysaccharide in rat pups. PLoS One 8:e78332
Roesler R, Lessa D, Venturella R, Vianna MR, Luft T, Henriques JA, Izquierdo I, Schwartsmann G (2004) Bombesin/gastrin-releasing peptide receptors in the basolateral amygdala regulate memory consolidation. Eur J Neurosci 19:1041–1045
Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, Martin JE (1997) Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome 8:711–713
Rosa RM, Flores DG, Appelt HR, Braga AL, Henriques JA, Roesler R (2003) Facilitation of long-term object recognition memory by pretraining administration of diphenyl diselenide in mice. Neurosci Lett 341:217–220
Sadowska AM, Manuel YKB, De Backer WA (2007) Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: discordant in vitro and in vivo dose-effects: a review. Pulm Pharmacol Ther 20:9–22
Sarter M, Parikh V (2005) Choline transporters, cholinergic transmission and cognition. Nat Rev Neurosci 6:48–56
Scaini G, Comim CM, Oliveira GM, Pasquali MA, Quevedo J, Gelain DP, Moreira JC, Schuck PF, Ferreira GC, Bogo MR, Streck EL (2013) Chronic administration of branched-chain amino acids impairs spatial memory and increases brain-derived neurotrophic factor in a rat model. J Inherit Metab Dis 36:721–730
Scaini G, de Rochi N, Jeremias IC, Deroza PF, Zugno AI, Pereira TC, Oliveira GM, Kist LW, Bogo MR, Schuck PF, Ferreira GC, Streck EL (2012a) Evaluation of acetylcholinesterase in an animal model of maple syrup urine disease. Mol Neurobiol 45:279–286
Scaini G, Jeremias GC, Furlanetto CB, Dominguini D, Comim CM, Quevedo J, Schuck PF, Ferreira GC, Streck EL (2014) Behavioral responses in rats submitted to chronic administration of branched-chain amino acids. JIMD Rep 13:159–167
Scaini G, Jeremias IC, Morais MO, Borges GD, Munhoz BP, Leffa DD, Andrade VM, Schuck PF, Ferreira GC, Streck EL (2012b) DNA damage in an animal model of maple syrup urine disease. Mol Genet Metab 106:169–174
Scaini G, Teodorak BP, Jeremias IC, Morais MO, Mina F, Dominguini D, Pescador B, Comim CM, Schuck PF, Ferreira GC, Quevedo J, Streck EL (2012c) Antioxidant administration prevents memory impairment in an animal model of maple syrup urine disease. Behav Brain Res 231:92–96
Scarr E, Dean B (2009) Role of the cholinergic system in the pathology and treatment of schizophrenia. Expert Rev Neurother 9:73–86
Schroder N (2003) Differential involvement of hippocampal and amygdalar NMDA receptors in contextual and aversive aspects of inhibitory avoidance memory in rats. J Clin Psychopharmacol 975:207–213
Sekhon B, Sekhon C, Khan M, Patel SJ, Singh I, Singh AK (2003) N-acetyl cysteine protects against injury in a rat model of focal cerebral ischemia. Brain Res 971:1–8
Sener RN (2005) Tyrosinemia: computed tomography, magnetic resonance imaging, diffusion magnetic resonance imaging, and proton spectroscopy findings in the brain. J Comput Assist Tomogr 29:323–325
Sgaravatti AM, Magnusson AS, de Oliveira AS, Rosa AP, Mescka CP, Zanin FR, Pederzolli CD, Wyse AT, Wannmacher CM, Wajner M, Dutra-Filho CS (2009) Tyrosine administration decreases glutathione and stimulates lipid and protein oxidation in rat cerebral cortex. Metab Brain Dis 24:415–425
Sgaravatti AM, Vargas BA, Zandona BR, Deckmann KB, Rockenbach FJ, Moraes TB, Monserrat JM, Sgarbi MB, Pederzolli CD, Wyse AT, Wannmacher CM, Wajner M, Dutra-Filho CS (2008) Tyrosine promotes oxidative stress in cerebral cortex of young rats. Int J Dev Neurosci 26:551–559
Silman I, Sussman JL (2005) Acetylcholinesterase: ‘classical’ and ‘non-classical’ functions and pharmacology. Curr Opin Pharmacol 5:293–302
Snyderman SE, Norton PM, Roitman E, Holt LE Jr (1964) Maple syrup urine disease, with particular reference to dietotherapy. Pediatrics 34:454–472
Soreq H, Seidman S (2001) Acetylcholinesterase-—new roles for an old actor. Nat Rev Neurosci 2:294–302
Sprong RC, Winkelhuyzen-Janssen AM, Aarsman CJ, van Oirschot JF, van der Bruggen T, van Asbeck BS (1998) Low-dose N-acetylcysteine protects rats against endotoxin-mediated oxidative stress, but high-dose increases mortality. Am J Respir Crit Care Med 157:1283–1293
Streck EL, De Pra SDT, Ferro PR, Carvalho-Silva M, Gomes LM, Agostini JF, Damiani A, Andrade VM, Schuck PF, Ferreira GC, Scaini G (2017) Role of antioxidant treatment on DNA and lipid damage in the brain of rats subjected to a chemically induced chronic model of tyrosinemia type II. Mol Cell Biochem 435(1-2):207–214. https://doi.org/10.1007/s11010-017-3070-5
Teodorak BP, Scaini G, Carvalho-Silva M, Gomes LM, Teixeira LJ, Rebelo J, De Pra SD, Zeni N, Schuck PF, Ferreira GC, Streck EL (2017) Antioxidants reverse the changes in energy metabolism of rat brain after chronic administration of L.-tyrosine. Metab Brain Dis 32:557–564
Tsukagoshi H, Morita T, Hitomi S, Saito S, Kadoi Y, Uchihashi Y, Kuribara H, Goto F (2000) Long-term clomipramine treatment upregulates forebrain acetylcholine muscarinic receptors, and reduces behavioural sensitivity to scopolamine in mice. J Pharm Pharmacol 52:87–92
Wu D, Hersh LB (1994) Choline acetyltransferase: celebrating its fiftieth year. J Neurochem 62:1653–1663
Zafarullah M, Li WQ, Sylvester J, Ahmad M (2003) Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci 60:6–20
Zribi H, Souissi A, Azzouz H, Tebib N, Mokni M (2016) Richner-Hanhart syndrome. Presse Med 45:264–265
Zugno AI, Chipindo H, Canever L, Budni J, Alves de Castro A, Bittencourt de Oliveira M, Heylmann AS, Gomes Wessler P, da Rosa SF, Damazio LS, Mastella GA, Kist LW, Bogo MR, Quevedo J, Gama CS (2015) Omega-3 fatty acids prevent the ketamine-induced increase in acetylcholinesterase activity in an animal model of schizophrenia. Life Sci 121:65–69
Funding
This research was supported by grants from Programa de Pós-graduação em Ciências da Saúde – Universidade do Extremo Sul Catarinense (UNESC) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experimental procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals issued by the National Institutes of Health and received an approval from the Ethics Committee of the Universidade do Extremo Sul Catarinense.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gomes, L.M., Scaini, G., Carvalho-Silva, M. et al. Antioxidants Reverse the Changes in the Cholinergic System Caused by L-Tyrosine Administration in Rats. Neurotox Res 34, 769–780 (2018). https://doi.org/10.1007/s12640-018-9866-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-018-9866-6