Abstract
Purpose
Historically, cardiac surgery patients have often been managed with supraphysiologic intraoperative oxygen levels to protect against the risks of cellular hypoxia inherent in the un-physiologic nature of surgery and cardiopulmonary bypass. This may result in excessive reactive oxygen species generation and exacerbation of ischemia-reperfusion injury. In this review, we synthesize all available data from randomized controlled trials (RCTs) to investigate the impact that hyperoxia has on postoperative organ dysfunction, length of stay, and mortality during adult cardiac surgery.
Source
We searched Medline, Embase, Scopus, and Cochrane Central Register of Controlled Trials databases using a high-sensitivity strategy for RCTs that compared oxygenation strategies for adult cardiac surgery. Our primary outcome was postoperative organ dysfunction defined by postoperative increases in myocardial enzymes, acute kidney injury, and neurologic dysfunction. Secondary outcomes were mortality, ventilator days, and length of stay in the hospital and intensive care unit.
Principal findings
We identified 12 RCTs that met our inclusion criteria. Risk of bias was unclear to high in all but one trial. Significant heterogeneity in timing of the treatment period and the oxygenation levels targeted was evident and precluded meta-analysis. The large majority of trials found no difference between hyperoxia and normoxia for any outcome. Two trials reported reduced postoperative myocardial enzymes and one trial reported reduced mechanical ventilation time in the normoxia group.
Conclusions
Hyperoxia had minimal impact on organ dysfunction, length of stay, and mortality in adult cardiac surgery. The current evidence base is small, heterogeneous, and at risk of bias.
Trial registration
International Prospective Register of Systematic Reviews (PROSPERO) (CRD42017074712). Registered 17 August 2017.
Résumé
Objectif
Historiquement, les patients de chirurgies cardiaques ? ont souvent été gérés avec une oxygénothérapie peropératoire supraphysiologique pour protéger contre le risque d’hypoxie cellulaire inhérent à la nature non physiologique de la chirurgie et de la circulation extracorporelle. Ceci peut entraîner la formation de dérivés réactifs de l’oxygène et l’exacerbation des lésions d’ischémie-reperfusion. Dans cette synthèse, nous avons résumé toutes les données disponibles provenant d’essais contrôlés randomisés (ECR) pour étudier l’impact de l’hyperoxie sur les troubles fonctionnels postopératoires des organes, la durée de séjour et la mortalité au cours de la chirurgie cardiaque de l’adulte.
Source
Nous avons fait des recherches dans les bases de données MEDLINE, Embase, Scopus et dans le Registre central Cochrane des essais contrôlés en utilisant une stratégie de grande sensibilité pour trouver les ECR comparant les stratégies d’oxygénation au cours de la chirurgie cardiaque de l’adulte. Notre principal critère d’évaluation était le dysfonctionnement postopératoire des organes défini par une augmentation postopératoire des enzymes myocardiques, une lésion rénale aiguë et une atteinte neurologique. Les critères d’évaluation secondaires étaient la mortalité, le nombre de jours de ventilation et la durée de séjour en unité de soins intensifs et à l’hôpital.
Constatations principales
Nous avons identifié 12 essais cliniques randomisés qui satisfaisaient nos critères d’inclusion. Le risque de biais allait d’indéterminé à élevé dans 11 des 12 essais. Une hétérogénéité significative dans l’horaire de la période de traitement et des taux d’oxygénation visés a été évidente et a empêché une méta-analyse. Dans leur grande majorité, aucune différence sur les critères d’évaluation n’a été trouvée dans les essais entre l’hyperoxie et la normoxie. Deux essais ont décrit une baisse des enzymes myocardiques en post opératoire et un essai a décrit une baisse de la durée de la ventilation mécanique dans le groupe normoxie.
Conclusions
L’hyperoxie a eu des répercussions minimes sur le dysfonctionnement des organes, la durée de séjour et la mortalité au cours de la chirurgie cardiaque de l’adulte. La base actuelle des données probantes est limitée, hétérogène et sujette à des biais.
Enregistrement de l’essai clinique
International Prospective Register of Systematic Reviews (PROSPERO) (CRD42017074712). Enregistré le 17 août 2017.
Similar content being viewed by others
Each year, more than 1.25 million patients worldwide undergo cardiac surgery utilizing cardiopulmonary bypass (CPB).1 The associated morbidity and mortality remain relatively high despite advances in surgical technique and anesthetic management.1,2,3 One of the principal goals in the perioperative care of the cardiac surgery patient is to maintain end-organ perfusion and tissue oxygenation. With this aim in mind, it has been common practice to provide supraphysiologic levels of oxygen to patients while on CPB to protect against the risks of cellular hypoxia inherent in the un-physiologic nature of surgery and CPB. Hyperoxia during CPB may also reduce gas microembolism and improve the oxidative killing function of neutrophils.4,5,6 The use of hyperoxia as an ischemic-preconditioning stimulus for cerebral and myocardial protection has also been described.7,8
Despite these potential benefits, there is an evolving understanding that hyperoxia may also have harmful systemic consequences.9 The proposed harm from hyperoxia in the setting of cardiac surgery using CPB can be broadly divided as arising from three separate but interrelated mechanisms. These mechanisms are hyperoxia-related cardiovascular dysregulation, direct tissue injury from increased production of reactive oxygen species (ROS), and enhancement of ischemia-reperfusion injury.9,10,11,12
High partial pressures of oxygen have multiple direct effects on the heart and peripheral vasculature. Oxygen has a direct vasoconstrictive effect that leads to an increase in systemic vascular resistance as well as an increased coronary vascular resistance.10,13,14 Hyperoxic conditions also lead to an impairment of diastolic dysfunction by altering calcium handling in the myocyte sarcoplasmic reticulum.15 These direct effects have the potential to result in impaired coronary and systemic perfusion during cardiac surgery. Hyperoxic conditions also result in the increased and incomplete reduction of oxygen leading to formation of the superoxide radical.4 Superoxide dismutases convert superoxide to hydrogen peroxide, which can go on to form a group of highly reactive oxygen-containing compounds known as ROS.
During cardiac surgery utilizing CPB, reperfusion of ischemic myocardium occurs after release of the aortic cross-clamp. The reperfusion phase is characterized by enhanced production of ROS, dysregulation of intracellular and mitochondrial calcium, microvascular dysfunction, and a hyperactive immune response.16 This detrimental constellation of events is known as ischemia-reperfusion injury and is believed to be exacerbated by hyperoxic conditions.4,17
There are compelling arguments both for and against the use of hyperoxia in cardiac surgery. The aim of this systematic review is to identify, critically appraise, and synthesize the highest quality evidence to inform clinical practice and identify unanswered questions for future trials. Our primary objective was to determine the impact of hyperoxic vs normoxic oxygenation strategies on postoperative organ dysfunction, length of stay, and mortality during adult cardiac surgery with CPB.
Methods
This systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO) on 17 August 2017 (CRD42017074712, http://www.crd.york.ac.uk/prospero/display_record.php?RecordID=74712). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was followed while writing this review.18
Eligibility criteria
We included randomized trials in humans ≥ 18 yr of age who underwent cardiac surgery, with at least 80% of patients undergoing CPB. This allowed for inclusion of trials that may have a small subset of patients that underwent off-pump cardiac surgery.
Only randomized controlled trials (RCTs) were considered for inclusion. In considering RCTs, we allowed for any intervention that targeted a specific partial pressure of oxygen (PaO2), or specified the delivered fraction of inspired oxygen (FiO2), at any point during the patient’s time in the operating room. We included any comparator PaO2 target or FiO2. Given that there is no universally accepted definition of hyperoxia,19 we did not pre-specify thresholds for hyperoxic or normoxic FiO2 or PaO2 targets, but instead relied on the individual study definitions.1,17
The primary outcome examined was postoperative organ dysfunction defined by: 1) postoperative increases in myocardial enzymes (creatine kinase [CK] and/or troponin), 2) acute kidney injury (AKI), and 3) neurologic dysfunction. The definition of AKI and neurologic dysfunction was in accordance with the individual study report definitions. Secondary outcomes included mortality at the longest follow-up, days on a ventilator, as well as hospital and intensive care unit (ICU) length of stay.
Information sources and search
A systematic search of the literature identified potentially relevant studies. The initial bibliographic database search strategy was designed by a professional librarian using Ovid Medline and was peer-reviewed by an independent librarian using the Peer Review of Electronic Search Strategies (PRESS) guidelines.20 Searches of the following bibliographic databases, from inception to present, were conducted on 26 June 2017 and then updated on 22 November 2017: Medline (Ovid), Embase (Ovid), Scopus (Elsevier), and the Cochrane Central Register of Controlled Trials (Wiley). Results were limited to English language publications. Animal studies, commentaries, editorials, letters, and historical articles were excluded. See the Appendix for the complete search strategy using Medline, which was subsequently adapted for the other databases. A search of the grey literature to identify unpublished studies was also conducted in clincialtrials.gov, the World Health Organization International Clinical Trials Registry Platform (ICTRP), and Open Grey on 26 June 2017 and again on 22 November 2017.
The electronic search was further supplemented with a hand search of conference abstracts presented in the past three years from the American Society of Anesthesiologists, the European Society of Anaesthesiology, the Canadian Anesthesiologists’ Society, the Society of Thoracic Surgeons, The American Association for Thoracic Surgery, The European Society of Thoracic Surgeons, and the Society of Critical Care Medicine. The reference lists of included articles were also mined for additional citations.
We included all English language reports regardless of their age or publication status. Both abstracts and full manuscripts were included. When only an abstract was available, we contacted the authors to request a full manuscript. All authors of completed trials identified through clinicaltrials.gov or ICTRP were contacted and asked to provide a manuscript for review.
Study selection
Titles and abstracts of the reports identified by our search were screened independently by two reviewers (J.H. and C.L.). Full texts were obtained for all relevant reports and were again independently screened for inclusion. Discrepancies between the two reviewer’s decisions were resolved by consensus discussion and consultation with a third reviewer (H.G.).
Data collection and processing
We developed a standardized data extraction form that was tested and revised by a single reviewer (J.H.). The form was then applied independently in duplicate by two reviewers (J.H. and C.L.) to all the included reports. The forms were reviewed and discrepancies resolved by consensus discussion.
Data extracted from the reports included first author and citation, study period, and funding source. Demographic details of the included patients in each trial were collected including age, body mass index, and sex. Information regarding the surgical procedures performed in each study included type of cardiac surgery performed, procedure urgency, CPB time, and aortic cross-clamp time. Details of the intervention and comparator in each trial were recorded, including the PaO2 levels or delivered FiO2, as well as the timing of the intervention. Finally, data were collected for all three primary and four secondary outcomes. This included information on how outcomes were defined by study authors, when outcomes were assessed, what assays were used, and from which sites blood samples were taken. If postoperative myocardial enzyme levels were sampled at multiple time points, we included their maximum value in our data set. We preferentially reported data for enzymes sampled from peripheral venous and arterial sites over those collected from coronary sinus blood.
Risk of bias
Risk of bias in the included studies was assessed using the Cochrane Risk of Bias Tool.21 Each report was assessed at the study level for risk of bias in seven domains including: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. Each study was evaluated independently by two reviewers (J.H. and C.L.). Disagreement between the two reviewer’s assessments was resolved by consensus discussion and consultation with a third reviewer (A.A.). Overall risk of bias for an individual study was considered high if there was a high risk of bias in any of the individual domains. If there were no domains at high risk of bias, but one or more domains had an unclear risk, the overall risk of bias was rated unclear. Only if all the domains were at low risk of bias was the study assessed as having a low risk of bias overall.
Synthesis of results
We intended to perform a meta-analysis for outcome if there was sufficient clinical and statistical homogeneity; the methods for a meta-analysis were outlined prospectively in our protocol. After all included trials had been reviewed, we determined that there was too much clinical heterogeneity to conduct a sound meta-analysis. In lieu of a meta-analysis, a narrative synthesis was performed. Further, since we did not conduct a meta-analysis, we were unable to formally investigate publication bias.
Results
Study characteristics
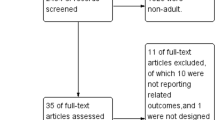
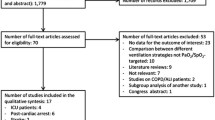
Our initial database search identified 4,421 items (Fig. 1). Following screening, we identified 11 RCTs that met our inclusion criteria. An additional trial was identified by hand searching the reference lists of the included RCTs, leaving us with a total of 12 RCTs for inclusion.22 Hand screening of conference abstracts did not identify any additional RCTs that were eligible for inclusion. Authors of an English abstract from a Korean-language manuscript were contacted for an English manuscript, but did not reply.23 There was sufficient detail in the abstract alone to allow for inclusion in our review. In two trials identified in our database search, details of the study intervention were reported with insufficient depth to be evaluated as part of our review and were not included.24,25 The authors were contacted for clarification, but we did not receive a response.
The 12 included RCTs were published between 1991 and 2016 (Table 1). Only five of the reports described how the trials were funded.1,8,9,26,27 None of these trials were industry supported. The average age of patients included in trial intervention groups ranged from 47 to 68 years (Table 1). The majority of patients were male in the treatment and control arms of all but one included trial (Table 1).
All of the RCTs included only patients scheduled for elective surgery. Nine of the trials included patients for coronary artery bypass grafting (CABG) only; one trial included patients for isolated valvular surgery only; two trials included patients for CABG, isolated valvular, as well as combined procedures (Table 1). Mean (standard deviation [SD]) CPB times were reported in ten trials and ranged from 63 (26) to 156 (10) min.1,9,22,23,26,27,28,29,30,31 Mean (SD) aortic cross clamp times were reported in ten trials and ranged from 43 (12) to 112 (43) min.1,8,9,22,23,24,26,28,29,31
There was marked heterogeneity in the timing of the study intervention in these trials. There were five distinct time periods over which the oxygenation strategy being evaluated was applied. These included the pre-CPB period, during CPB, during the reperfusion phase (i.e., after aortic cross-clamp removal), during rewarming, and during the postoperative ICU period. The 12 included RCTs applied their intervention over six distinct combinations of these time periods (Table 1).
The specific oxygenation strategy used in the normoxia and hyperoxia arms of each study also varied substantially. Six trials used specific PaO2 targets, five trials prescribed a set FiO2, and one trial used a combination of PaO2 and pulse oximetry (SpO2) targets (Table 1). There were also significant differences in authors’ choices of oxygenation thresholds for their normoxic and hyperoxic treatment arms (Figs. 2 and 3).
The ranges of fraction of inspired oxygen (FiO2) prescribed to the normoxic and hyperoxic treatment groups in individual trials included in our systematic review. Red boxes represent hyperoxic groups and blue boxes represent normoxic. Toraman et al.32 included three treatment groups
The resulting heterogeneity from the differences in timing of the study intervention as well as the varying oxygenation strategies precluded us from conducting a meta-analysis.
Risk of bias
Risk of bias assessment using the Cochrane Risk of Bias Tools is presented in Fig. 4. Only one report described methods of sequence generation and allocation concealment that were rigorous and detailed enough to be at low risk of bias.1 In seven studies the risk of bias was unclear. Four studies were at high risk of bias for selective reporting.22,26,30,32 These studies reported on outcomes that were not detailed in a protocol or their methods section. Reporting quality was generally poor.
Risk of bias summary. Each study was assessed at the report level using the Cochrane Risk of Bias Tool. Assessments were done in duplicate by two blinded reviewers. Discrepancies were resolved by consensus and consultation with a third reviewer. Seven unique domains were assessed. Green circles represent a low risk of bias, yellow represent an unclear risk of bias, and red represent a high risk of bias. The overall assessment was based on the highest risk assessment in any individual domain
Primary outcomes
Five trials reported on postoperative CK levels (Table 2). One trial found that mean (SD) post-CPB coronary sinus blood CK levels were 293 (21) U·L−1 when patients had a PaO2 target of 140 mmHg on CPB and 672 (130) U·L−1 when a PaO2 of 400 mmHg was targeted (P = 0.002).30 The other four trials reported on the CK-MB subtype in arterial blood and found no significant difference in postoperative levels between groups.8,9,28,29
Six trials included postoperative troponin levels as a reported outcome (Table 2). One trial found that the maximum postoperative troponin was significantly higher in the hyperoxia group.29 Five trials found no significant difference between postoperative troponins in the hyperoxic and near-physiologic groups.1,8,9,23,26
The occurrence of postoperative AKI was examined in three trials (Table 2). The definitions of AKI in the various reports varied.33,34 One trial used the Kidney Disease: Improving Global Outcomes AKI Work Group guidelines (KDIGO), while the other two trials reported on “renal failure” and “renal complications’ without further details.1,31,32 One trial found that 8.3% of patients on CPB with a PaO2 of 190-300 mmHg had postoperative renal failure whereas 4.2% of patients exposed to a PaO2 of 75-112 mmHg had renal failure.31 A P value was not reported. One trial found no significant difference in postoperative AKI in hyperoxic and near-physiologic groups (using KDIGO criteria); another had no postoperative renal complications in either group.1,32
Neurologic dysfunction was examined in two trials (Table 2). In a trial comparing CPB with a PaO2 of 190-300 mmHg to 75-112 mmHg, 4.2% of patients in the hyperoxic group had a cerebrovascular accident compared with 0% in the near-physiologic group (no P value reported).31 In another trial there was no postoperative neurologic dysfunction reported across any groups.32
Secondary outcomes
Postoperative mechanical ventilation time was included as an outcome in six trials (Table 2). In one trial patients who were exposed to a PaO2 of 75-112 mmHg on CPB were ventilated for a mean (SD) of 5.3 (1.8) hr, while those with a PaO2 of 190-300 mmHg required 7.2 (2.5) hr of mechanical ventilation (P < 0.01).31 The other five trials reported no difference between groups.1,9,27,28,30
Five RCTs examined length of stay in the ICU and four examined length of stay in the hospital (Table 2). The oxygenation strategy had no impact on either outcome in any study. There was no difference in mortality between groups in any of the six RCTs that included it as an outcome (Table 2).
Discussion
The level of oxygen that patients should be exposed to during cardiac surgery is an important clinical question that has been asked by investigators for over two decades. What makes this question so intriguing is the simplicity of the intervention, the complexity of its multisystem effects, and the potential to make a real difference to clinically important outcomes.35,36 Our systematic review aimed to assess the impact that hyperoxia during adult cardiac surgery has on postoperative organ dysfunction, length of stay, and mortality by synthesizing the evidence from all available RCTs to date. We were unable to perform a meta-analysis because of the significant clinical heterogeneity in the included trials and instead provide this narrative synthesis.
We chose to examine postoperative organ dysfunction as our primary outcome because the impact of hyperoxia during CPB on ROS production and ischemia-reperfusion injury is best detected at the individual organ level. Although it could be argued that postoperative myocardial enzyme levels are not a particularly patient-centred outcome, we believe that it is an important marker of myocardial injury.37 Additionally, postoperative myocardial enzyme levels are the most commonly studied outcome in this field and therefore inclusion of these results allowed us to synthesize the most comprehensive summary of the current evidence. We anticipated that report definitions and timing of data collection for neurologic dysfunction, AKI, and rise in myocardial enzymes would be heterogeneous. Therefore, we chose to summarize individual report definitions rather than dictate our own a priori. Mortality, days on a ventilator, and length of stay (ICU and hospital) were also included as secondary outcomes, ensuring that all outcomes important to individual patients were addressed.
The RCTs that met the inclusion criteria for our review spanned over two decades (1991 to 2016).1,9,31 There was significant heterogeneity in study design, particularly in two key areas: the timeline over which the oxygenation strategy being studied was employed and the degree of hyperoxia or normoxia participants were exposed to. Additionally, some trials subjected participants to a constant FiO2 whereas others adjusted FiO2 to meet a PaO2 target.
An important limitation to interpreting this literature is that the PaO2 targets were so disparate across studies that the normoxic targets in some trials were higher than the hyperoxic targets in others (Figs. 2 and 3). One explanation for this is that there are no established guidelines for specific PaO2 targets on bypass and clinical practice is highly variable.38 The results of our review suggest that there is no agreement in the literature as to what conditions should be considered normoxic or hyperoxic during cardiac surgery. That said, a trend towards lower PaO2 targets in the two most recent RCTs may be indicative of a recent change in clinical practice towards more conservative oxygenation strategies based on evidence from the myocardial infarction and cardiac arrest literature suggesting that high oxygen levels during reperfusion after ischemia may have deleterious effects.39,40,41,42 Real-time continuous in-line PaO2 monitoring has only recently been widely available, which may explain why some investigators chose to prescribe a specific FiO2 to their intervention groups rather than set PaO2 targets. This variation in study design further made it impossible to proceed with a rigorous meta-analysis of trial data.
The majority of trials found no difference in postoperative organ dysfunction between groups treated with hyperoxic and normoxic strategies during cardiac surgery. One small trial of 20 patients undergoing elective CABG found that the peak postoperative troponin levels were higher in patients who were exposed to a hyperoxic strategy during reperfusion.29 Notably, there was no difference in the peak CK-MBs between groups in this same trial. Another trial of 40 patients having elective CABG surgery found post-CPB CK levels were higher in the coronary sinus blood of patients who had a higher PaO2 target during CPB.30 Only two trials have shown a statistically significant result for any of our primary outcomes. Together these two positive trials only contained 60 of the 741 patients studied in trials that reported on at least one of the primary outcomes.
There was no difference between the hyperoxic and normoxic intervention groups for all secondary outcomes in all but one trial. One RCT found that patients exposed to a higher PaO2 on CPB spent a longer time on the ventilator postoperatively.31 No trials found that the oxygenation strategy used during cardiac surgery had a significant impact on mortality or length of stay.
Risk of bias, as assessed using the Cochrane Risk of Bias Tool, was low only in one trial.1 Four studies had a high risk of bias because they reported on outcomes that were not described in their protocol or methods.22,26,30,32 Seven studies had an unclear risk of bias, primarily because they did not report on their methods of sequence generation or allocation concealment with enough detail to satisfy the Cochrane criteria for low risk of bias.8,9,23,28,29,31 That said, the primary and secondary outcomes included in our review –-were hard outcomes unlikely to be impacted by lack of blinding of participants, personnel, and outcome assessors.43 Therefore, we did not require strict blinding to obtain a low risk of bias assessment for these domains. It would be helpful if authors of future trials would closely adhere to the CONSORT statement while writing their reports to provide better clarity for risk of bias assessment.44 Our search strategy and inclusion criteria were limited to English-language reports, which may have introduced a review-level selection bias.
It is unclear why avoidance of hyperoxia in cardiac surgery has not translated into improved clinical outcomes. It is a complex question with an answer that is perhaps too nuanced to be elucidated by any of the trials published to date. If the central harm of hyperoxia is due to enhanced ROS production resulting in exacerbation of ischemia reperfusion injury, then its clinical impact must be consistent with the timing and proportional to the actual degree of ischemia. Trials such as those published by McGuinness et al.—by far the largest included in this review—that delivered near-physiologic oxygen levels may have actually aggravated the occurrence of CPB-related ischemia by exposing patients to the relatively lower oxygen levels during time periods of poor tissue perfusion.1 It follows then that those in the arm that received hyperoxia could have reduced ischemic injury during CPB and therefore reduced ischemia-reperfusion injury in the post-reperfusion period.1 If there is reduced ischemia in the first place (i.e., in the hyperoxia group, which was originally hypothesized to be injurious), there is less basis for ischemia-reperfusion injury and the amount of oxygen that the patient receives during reperfusion becomes less relevant.
An additional reason for the lack of impact of hyperoxia avoidance on clinical outcomes may be that the treatment separation achieved between hyperoxic and normoxic groups in some trials was quite narrow or non-existent. The largest of the trials by McGuinness et al. failed to achieve treatment separation in the pre- and post-CPB intervention periods.1 This might be explained by the trial’s pragmatic design and anesthesiologists who were uncomfortable targeting a near-physiologic PaO2 while patients were not on CPB. Clinicians and trialists should be reassured by the results of the other most recently published trial by Smit et al.9 This group achieved excellent treatment separation between the normoxic and hyperoxic groups in all phases of their study and had no hypoxic events (PaO2 < 55 mmHg).9
We speculate that the ideal strategy may be to exploit temporal variation in PaO2 during cardiac surgery to improve clinical outcomes. That is, avoidance of hyperoxia is likely to be of most benefit during the reperfusion phase after resumption of pulsatile flow to ischemic tissue beds. On the other hand, use of hyperoxia during periods of poor tissue perfusion might be beneficial compared with normoxia. Thus, future trials could target hyperoxia in the pre-bypass period and while on CPB to limit ischemia and then target normoxia during reperfusion to reduce exacerbation of subsequent ROS-mediated reperfusion injury. There are currently at least four registered trials in progress that would have met inclusion for this review and will enhance our understanding of the topic when complete.45,46,47,48 Nevertheless, none of these trials employ oxygenation targets that vary at critical points during the procedure to minimize both tissue hypoxia and ischemia-reperfusion injury.
Conclusions
Compared with normoxic strategies, the impact of hyperoxia on postoperative organ dysfunction, length of stay, and mortality in adult cardiac surgery patients appears to have been minimal, though the evidence base is highly heterogeneous (with marked variability in study design) and is also at risk of bias. There is no universal consensus on what represents a truly hyperoxic PaO2 during cardiac surgery. Clinical equipoise exists for future trials to expose patients to a wide range of oxygenation strategies. Further rigorously designed RCTs with meticulous reporting are needed to inform decision-making.
References
McGuinness SP, Parke RL, Drummond K, et al. A multicenter, randomized, controlled phase IIb trial of avoidance of hyperoxemia during cardiopulmonary bypass. Anesthesiology 2016; 125: 465-73.
Scheinerman SJ, Dlugacz YD, Hartman AR, et al. Journey to top performance: a multipronged quality improvement approach to reducing cardiac surgery mortality. Jt Comm J Qual Patient Saf 2015; 41: 52-61.
Ghanta RK, LaPar DJ, Zhang Q, et al. Obesity increases risk-adjusted morbidity, mortality, and cost following cardiac surgery. J Am Heart Assoc 2017; 6: e003831.
Young RW. Hyperoxia: a review of the risks and benefits in adult cardiac surgery. J Extra Corpor Technol 2012; 44: 241-9.
Nollert G, Nagashima M, Bucerius J, Shin’oka T, Jonas RA. Oxygenation strategy and neurologic damage after deep hypothermic circulatory arrest. I. Gaseous microemboli. J Thorac Cardiovasc Surg 1999; 117: 1166-71.
Qadan M, Battista C, Gardner SA, Anderson G, Akca O, Polk HC Jr. Oxygen and surgical site infection: a study of underlying immunologic mechanisms. Anesthesiology 2010; 113: 369-77.
Liu W, Liu K, Tao H, Chen C, Zhang JH, Sun X. Hyperoxia preconditioning: the next frontier in neurology? Neurol Res 2012; 34: 415-21.
Karu I, Loit R, Zilmer K, et al. Pre-treatment with hyperoxia before coronary artery bypass grafting - effects on myocardial injury and inflammatory response. Acta Anaesthesiol Scand 2007; 51: 1305-13.
Smit B, Smulders YM, de Waard MC, et al. Moderate hyperoxic versus near-physiological oxygen targets during and after coronary artery bypass surgery: a randomised controlled trial. Crit Care 2016. DOI: https://doi.org/10.1186/s13054-016-1240-6.
Thomson AJ, Drummond GB, Waring WS, Webb DJ, Maxwell SR. Effects of short-term isocapnic hyperoxia and hypoxia on cardiovascular function. J Appl Physiol 1985; 2006(101): 809-16.
Spoelstra-de Man AM, Smit B, Oudemans-van Straaten HM, Smulders YM. Cardiovascular effects of hyperoxia during and after cardiac surgery. Anaesthesia 2015; 70: 1307-19.
Ansley DM, Raedschelders K, Choi PT, Wang B, Cook RC, Chen DD. Propofol cardioprotection for on-pump aortocoronary bypass surgery in patients with type 2 diabetes mellitus (PRO-TECT II): a phase 2 randomized-controlled trial. Can J Anesth 2016; 63: 442-53.
Farquhar H, Weatherall M, Wijesinghe M, et al. Systematic review of studies of the effect of hyperoxia on coronary blood flow. Am Heart J 2009; 158: 371-7.
Anderson KJ, Harten JM, Booth MG, et al. The cardiovascular effects of normobaric hyperoxia in patients with heart rate fixed by permanent pacemaker. Anaesthesia 2010; 65: 167-71.
Mak S, Azevedo ER, Liu PP, Newton GE. Effect of hyperoxia on left ventricular function and filling pressures in patients with and without congestive heart failure. Chest 2001; 120: 467-73.
Frank A, Bonney M, Bonney S, Weitzel L, Koeppen M, Eckle T. Myocardial ischemia reperfusion injury: from basic science to clinical bedside. Semin Cardiothorac Vasc Anesth 2012; 16: 123-32.
Helmerhorst HJ, Schultz MJ, van der Voort PH, de Jonge E, van Westerloo DJ. Bench-to-bedside review: the effects of hyperoxia during critical illness. Crit Care 2015; 19: 284.
Moher D, Liberati A, Tetzlaff J. Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535.
Hayes RA, Shekar K, Fraser JF. Is hyperoxaemia helping or hurting patients during extracorporeal membrane oxygenation? Review of a complex problem. Perfusion 2013; 28: 184-93.
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 2016; 75: 40-6.
Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from URL: http://handbook.cochrane.org (accessed February 2018).
Abdel-Rahman U, Aybek T, Moritz A, Kleine P, Matheis G. Graded reoxygenation limits lipid peroxidation during surgical reperfusion. Med Sci Monit 2003; 9: CR389-91.
Kim KB, Choi SC, Choi KL, et al. Comparison of inflammatory response and myocardial injury between normoxic and hyperoxic condition during cardiopulmonary bypass. Korean J Thorac Cardiovasc Surg 2001; 34: 524-33.
Ihnken K, Winkler A, Beyersdorf, et al. Reduction of oxidative damage and nitric oxide on cardiopulmonary bypass by controlling pO2 during open heart surgery. Circulation 1995; 92(Suppl I): I-763-4 (abstract).
Mandel IA, Mikheev SL, Podoksenov YK, Svirko YS, Suhodolo IV, Shipulin VM. Hypoxic-hyperoxic preconditioning: a novel technique for myocardial protection against ischemia-reperfusion injury in coronary surgery. Eur J Heart Fail 2017; 19: 349 (abstract).
Karu I, Tahepold P, Ruusalepp A, Zilmer K, Zilmer M, Starkopf J. Effects of 60 minutes of hyperoxia followed by normoxia before coronary artery bypass grafting on the inflammatory response profile and myocardial injury. J Negat Results Biomed 2012; 11: 14.
Pizov R, Weiss YG, Oppenheim-Eden A, et al. High oxygen concentration exacerbates cardiopulmonary bypass-induced lung injury. J Cardiothorac Vasc Anesth 2000; 14: 519-23.
Lee JS, Kim JC, Chung JY, Wong SW, Choi KH, Kwak YL. Effect of arterial oxygen tension during reperfusion on myocardial recovery in patients undergoing valvular heart surgery. Korean J Anesthesiol 2010; 58: 122-8.
Inoue T, Ku K, Kaneda T, Zang Z, Otaki M, Oku H. Cardioprotective effects of lowering oxygen tension after aortic unclamping on cardiopulmonary bypass during coronary artery bypass grafting. Circ J 2002; 66: 718-22.
Ihnken K, Winkler A, Schlensak C, et al. Normoxic cardiopulmonary bypass reduces oxidative myocardial damage and nitric oxide during cardiac operations in the adult. J Thorac Cardiovasc Surg 1998; 116: 327-34.
Belboul A, Al-khaja N, Ericson C, et al. The effect of hyperoxia during cardiopulmonary bypass on blood cell rheology and postoperative morbidity associated with cardiac surgery. J Extra Corpor Technol 1991; 23: 43-8.
Toraman F, Evrenkaya S, Senay S, Karabulut H, Alhan C. Adjusting oxygen fraction to avoid hyperoxemia during cardiopulmonary bypass. Asian Cardiovasc Thorac Ann 2007; 15: 303-6.
Shaw AD. Cardiac surgery-associated acute kidney injury: tools for enriching clinical trial populations. Can J Anesth 2017; 64: 793-6.
Karkouti K, Rao V, Chan CT. Wijeysundera DN; TACS Investigators. Early rise in postoperative creatinine for identification of acute kidney injury after cardiac surgery. Can. J Anesth 2017; 64: 801-9.
Wijeysundera DN. Predicting outcomes: is there utility in risk scores? Can J Anesth 2016; 63: 148-58.
Myles PS. Perioperative outcomes: are we asking the right questions? Can J Anesth 2016; 63: 138-41.
Royo MB, Fleisher LA. Chasing myocardial outcomes: perioperative myocardial infarction and cardiac troponin. Can J Anesth 2016; 63: 227-32.
Murphy GS, Hessel EA, Groom RC. Optimal perfusion during cardiopulmonary bypass: an evidence based approach. Anesth Analg 2009; 108: 1394-417.
Stub D, Smith K, Bernard S, et al. Air Versus oxygen in ST-segment elevation myocardial infarction. Circulation 2015; 131: 2143-50.
Janz DR, Hollenbeck RD, Pollock JS, McPherson JA, Rice TW. Hyperoxia is associated with increased mortality in patients treated with mild therapeutic hypothermia after sudden cardiac arrest. Crit Care Med 2012; 40: 3135-9.
Wang CH, Chang WT, Huang CH, et al. The effect of hyperoxia on survival following adult cardiac arrest: a systematic review and meta-analysis of observational studies. Resuscitation 2014; 85: 1142-8.
Hofmann R, James SK, Jernberg T, et al. Oxygen therapy in suspected acute myocardial infarction. N Engl J Med 2017; 377: 1240-9.
Wood L, Egger M, Gluud LL, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2008; 336: 601-5.
Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT. statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 2010(340): c332.
U.S. National Library of Medicine. ClinicalTrials.gov. Identifier NCT02361944. Risk of Oxygen During Cardiac Surgery Trial (ROCS) - 2017. Available from URL: https://clinicaltrials.gov/ct2/show/NCT02361944 (accessed February 2018).
U.S. National Library of Medicine. ClinicalTrials.gov. Identifier NCT02819739, Impact of Hyperoxia During Cardiopulmonary (CARDIOX) - 2016. Available from URL: https://clinicaltrials.gov/ct2/show/NCT02819739 (accessed February 2018).
Shaefi S, Marcantonio ER, Mueller A, et al. Intraoperative oxygen concentration and neurocognition after cardiac surgery: study protocol for a randomized controlled trial. Trials 2017; 18: 600.
National Library of Medicine (US). ClinicalTrials.gov.Identifier NCT02673931, GLP-1 and Hyperoxia for Organ Protection in Heart Surgery (GLORIOUS) - 2016. Available from URL: https://clinicaltrials.gov/ct2/show/NCT02673931 (accessed February 2018).
Acknowledgements
We thank Sarah Visintini, MLIS (Berkman Library, University of Ottawa Heart Institute), for peer review of the MEDLINE search strategy.
Conflicts of interest
None declared.
Editorial responsibility
This submission was handled by Dr. Gregory L. Bryson, Deputy Editor-in-Chief, Canadian Journal of Anesthesia.
Author contributions
Jeffrey Heinrichs, Hilary P. Grocott, and Ahmed Abou-Setta designed the review and wrote the protocol. Ahmed Abou-Setta provided methodologic advice. Christine Neilson designed and executed the search. Jeffrey Heinrichs and Carly Lodewyks acquired the data. Jeffrey Heinrichs, Carly Lodewyks, and Hilary P. Grocott analyzed and interpreted the data. Jeffrey Heinrichs drafted the manuscript. Hilary P. Grocott supervised the review and served as a content expert. All authors critically revised the article for important intellectual content.
Sources of support
The review was funded by the Department of Anesthesia, Perioperative, and Pain Medicine, Max Rady School of Medicine, University of Manitoba.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
APPENDIX Search Strategy for Medline (Ovid)
APPENDIX Search Strategy for Medline (Ovid)
Ovid MEDLINE(R) Epub Ahead of Print, In-Process, & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>
Date searched: 26 June 2017
1 | Hyperoxia/ | 3215 |
2 | Oxygen/ | 154591 |
3 | (hyperoxia or hyperoxias or hyperoxic or hyperox?emia or hyperoxygenat*).ti,ab,kf. | 8900 |
4 | (normoxemia or normoxia).ti,ab,kf. | 7847 |
5 | (oxygen adj5 (“partial pressure” or fraction or tension)).ti,ab,kf. | 20327 |
6 | ((oxygen or oxygenation) adj2 strategy).ti,ab,kf. | 111 |
7 | or/1-6 | 175013 |
8 | cardiopulmonary bypass/ | 22748 |
9 | (((Cardiopulmonary or “Cardio pulmonary” or “heart lung” or heartlung) adj3 bypass*) or CPB).ti,ab,kf. | 31454 |
10 | 8 or 9 | 38064 |
11 | 7 and 10 | 1636 |
12 | 11 not (exp animals/not humans/) | 1226 |
13 | Comment/ | 693182 |
14 | Editorial/ | 443105 |
15 | News/ | 183664 |
16 | (letter not (letter and randomized controlled trial)).pt. | 971042 |
17 | historical article/ | 348446 |
18 | or/13-17 | 2084513 |
19 | 12 not 18 | 1174 |
20 | limit 19 to English language | 1086 |
Rights and permissions
About this article
Cite this article
Heinrichs, J., Lodewyks, C., Neilson, C. et al. The impact of hyperoxia on outcomes after cardiac surgery: a systematic review and narrative synthesis. Can J Anesth/J Can Anesth 65, 923–935 (2018). https://doi.org/10.1007/s12630-018-1143-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-018-1143-x