Abstract
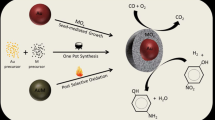
In this article, we review the recent progress and our research activity on the synthesis of inorganic shell nanostructures to enhance the catalytic performance and stability of metal nanoparticles in catalytic applications. First, we introduce general synthetic strategies for the fabrication of inorganic nanoscale shell layers, including template-assisted sol-gel coating, hydrothermal (or solvothermal) synthesis and the self-templating process. We also discuss recent examples of metal nanoparticles (NPs) with nanoscale shell layers, namely core–shell, yolk–shell and multiple NPs-embedded nanoscale shell. We then discuss the performance and stability of metal particles in practical catalytic applications. Finally, we conclude with a summary and perspective on the further progress of inorganic nanostructure with nanoscale shell layers for catalytic applications.















Similar content being viewed by others
References
Lee H, Habas SE, Kweskin S, Butcher D, Somorjai GA, Yang P. Morphological control of catalytically active platinum nanocrystals. Angew Chem Int Ed. 2006;45(46):7824.
Bratlie KM, Lee H, Komvopoulos K, Yang P, Somorjai GA. Platinum nanoparticle shape effects on benzene hydrogenation selectivity. Nano Lett. 2007;7(10):3097.
Lee I, Delbecq F, Morales R, Albiter MA, Zaera F. Tuning selectivity in catalysis by controlling particle shape. Nat Mater. 2009;8:132.
Lee I, Zaera F. Catalytic conversion of olefins on supported cubic platinum nanoparticles: selectivity of (100) versus (111) surfaces. J Catal. 2010;269(2):359.
Kim P, Joo JB, Kim H, Kim W, Kim Y, Song IK, Yi J. Preparation of mesoporous Ni–alumina catalyst by one-step sol–gel method: control of textural properties and catalytic application to the hydrodechlorination of o-dichlorobenzene. Catal Lett. 2005;104(3):181.
Kim P, Kim H, Joo JB, Kim W, Song IK, Yi J. Effect of nickel precursor on the catalytic performance of Ni/Al2O3 catalysts in the hydrodechlorination of 1,1,2-trichloroethane. J Mol Catal A Chem. 2006;256(1–2):178.
Kim P, Joo JB, Kim W, Kim J, Song IK, Yi J. Preparation of highly dispersed Pt catalyst using sodium alkoxide as a reducing agent and its application to the methanol electro-oxidation. J Mol Catal A Chem. 2007;263(1–2):15.
Joo JB, Kim P, Kim W, Yi J. Preparation and application of mesocellular carbon foams to catalyst support in methanol electro-oxidation. Catal Today. 2008;131(1–4):219.
Kim P, Joo JB, Kim W, Kim J, Song IK, Yi J. NaBH4-assisted ethylene glycol reduction for preparation of carbon-supported Pt catalyst for methanol electro-oxidation. J Power Sources. 2006;160(2):987.
Kim C, Lee H. Shape effect of Pt nanocrystals on electrocatalytic hydrogenation. Catal Commun. 2009;11(1):7.
Jia CJ, Schüth F. Colloidal metal nanoparticles as a component of designed catalyst. Phys Chem Chem Phys. 2011;13:2457.
Mahmoud MA, Tabor CE, El-Sayed MA, Ding Y, Wang ZL. A new catalytically active colloidal platinum nanocatalyst: the multiarmed nanostar single crystal. J Am Chem Soc. 2008;130(14):4590.
Taguchi A, Schüth F. Ordered mesoporous materials in catalysis. Micropor Mesopor Mat. 2005;77(1):1.
Li W, Wu Z, Wang J, Elzatahry AA, Zhao D. A perspective on mesoporous TiO2 materials. Chem Mater. 2014;26(1):287.
Davidson M, Ji Y, Leong GJ, Kovach NC, Trewyn BG, Richards RM. Hybrid mesoporous silica/noble-metal nanoparticle materials—synthesis and catalytic applications. ACS Appl Nano Mater. 2018;1(9):4386.
Zhang Q, Joo JB, Lu Z, Dahl M, Oliveira D, Ye M, Yin Y. Self-assembly and photocatalysis of mesoporous TiO2 nanocrystal clusters. Nano Res. 2011;4(1):103.
Park J, Song H. Metal@Silica yolk–shell nanostructures as versatile bifunctional nanocatalysts. Nano Res. 2011;4(1):33.
Zhang Q, Lee I, Joo JB, Zaera F, Yin Y. Core–shell nanostructured catalysts. Acc Chem Res. 2012;46(8):1816.
Chen D, Cao L, Huang F, Imperia P, Cheng YB, Caruso RA. Synthesis of monodisperse mesoporous titania beads with controllable diameter, high surface areas, and variable pore diameters (14–23 nm). J Am Chem Soc. 2010;132(12):4438.
Zhang Q, Ge J, Goebl J, Hu Y, Lu Z, Yin Y. Rattle-type silica colloidal particles prepared by a surface-protected etching process. Nano Res. 2009;2(7):583.
Yun HJ, Lee H, Joo JB, Kim W, Yi J. Influence of aspect ratio of TiO2 nanorods on the photocatalytic decomposition of formic acid. J Phys Chem C. 2009;113(8):3050.
Yin Y, Rioux RM, Erdonmez CK, Hughes S, Somorjai GA, Alivisatos AP. Formation of hollow nanocrystals through the nanoscale kirkendall effect. Science. 2004;304(5671):711.
Yang Z, Niu Z, Lu Y, Hu Z, Han CC. Templated synthesis of inorganic hollow spheres with a tunable cavity size onto core–shell gel particles. Angew Chem Int Ed. 2003;42(17):1943.
Joo JB, Dahl M, Li N, Zaera F, Yin Y. Tailored synthesis of mesoporous TiO2 hollow nanostructures for catalytic applications. Energ Environ Sci. 2013;6:2082.
Joo JB, Liu H, Lee YJ, Dahl M, Yu H, Zaera F, Yin Y. Tailored synthesis of C@TiO2 yolk–shell nanostructures for highly efficient photocatalysis. Catal Today. 2016;264(15):261.
Moon GD, Joo JB, Dahl M, Jung H, Yin Y. Nitridation and layered assembly of hollow TiO2 shells for electrochemical energy storage. Adv Funct Mater. 2014;24(6):848.
Liang X, Li J, Joo JB, Gutiérrez A, Tillekaratne A, Lee I, Yin Y, Zaera F. Diffusion through the shells of yolk–shell and core–shell nanostructures in the liquid phase. Angew Chem Int Ed. 2012;51(32):8034.
Li J, Liang X, Joo JB, Lee I, Yin Y, Zaera F. Mass transport across the porous oxide shells of core–shell and yolk–shell nanostructures in liquid phase. J Phys Chem C. 2013;117(39):20043.
Zhang Q, Zhang T, Ge J, Yin Y. Permeable silica shell through surface-protected etching. Nano Lett. 2008;8(9):2867.
Lee I, Joo JB, Yin Y, Zaera F. A Yolk@Shell nanoarchitecture for Au/TiO2 catalysts. Angew Chem Int Ed. 2011;50(43):10208.
Joo JB, Zhang Q, Dahl M, Zaera F, Yin Y. Synthesis, crystallinity control, and photocatalysis of nanostructured titanium dioxide shells. J Mater Res. 2013;28(3):362.
Lee H, Jeong U, Kim Y, Joo JB. Magnetically-separable and thermally-stable Au nanoparticles encapsulated in mesoporous silica for catalytic applications. Top Catal. 2017;60(9–11):763.
Jeong U, Joo JB, Kim Y. Au nanoparticle-embedded SiO2–Au@SiO2 catalysts with improved catalytic activity, enhanced stability to metal sintering and excellent recyclability. RSC Adv. 2015;5:55608.
Joo JB, Lee I, Dahl M, Moon GD, Zaera F, Yin Y. Controllable synthesis of mesoporous TiO2 hollow shells: toward an efficient photocatalyst. Adv Funct Mater. 2013;23(34):4246.
Joo JB, Zhang Q, Dahl M, Lee I, Goebl J, Zaera F, Yin Y. Control of the nanoscale crystallinity in mesoporous TiO2 shells for enhanced photocatalytic activity. Energ Environ Sci. 2012;5:6321.
Joo JB, Zhang Q, Lee I, Dahl M, Zaera F, Yin Y. Mesoporous anatase titania hollow nanostructures though silica-protected calcination. Adv Funct Mater. 2012;22(1):166.
Joo JB, Vu A, Zhang Q, Dahl M, Gu M, Zaera F, Yin Y. A sulfated ZrO2 hollow nanostructure as an acid catalyst in the dehydration of fructose to 5-hydroxymethylfurfural. ChemSusChem. 2013;6(10):2001.
Lou XW, Yuan C, Zhang Q, Archer LA. Platinum-functionalized octahedral silica nanocages: synthesis and characterization. Angew Chem Int Ed. 2006;45(23):3825.
Teo JJ, Chang Y, Zeng HC. Fabrications of hollow nanocubes of Cu2O and Cu via reductive self-assembly of CuO nanocrystals. Langmuir. 2006;22(17):7369.
Cao SW, Zhu YJ, Cheng GF, Huang YH. Preparation and photocatalytic property of α-Fe2O3 hollow core/shell hierarchical nanostructures. J Phys Chem Solids. 2010;71(12):1680.
Li N, Zhang Q, Liu J, Joo JB, Lee A, Gan Y, Yin Y. Sol–gel coating of inorganic nanostructures with resorcinol–formaldehyde resin. Chem Commun. 2013;49:5135.
Liu R, Mahurin SM, Li C, Unocic RR, Idrobo JC, Gao H, Pennycook SJ, Dai S. Dopamine as a carbon source: the controlled synthesis of hollow carbon spheres and yolk-structured carbon nanocomposites. Angew Chem Int Ed. 2011;50(30):6799.
Lee I, Joo JB, Yin Y, Zaera F. Au@Void@TiO2 yolk–shell nanostructures as catalysts for the promotion of oxidation reactions at cryogenic temperatures. Surf Sci. 2016;648:150.
Pastoriza-Santos I, Koktysh DS, Mamedov AA, Giersig M, Kotov NA, Liz-Marzán LM. One-pot synthesis of Ag@TiO2 core–shell nanoparticles and their layer-by-layer assembly. Langmuir. 2000;16(6):2731.
Mayya KS, Gittins DI, Dibaj AM, Caruso F. Nanotubes prepared by templating sacrificial nickel nanorods. Nano Lett. 2001;1(12):727.
Zhong Z, Yin Y, Gates B, Xia Y. Preparation of mesoscale hollow spheres of TiO2 and SnO2 by templating against crystalline arrays of polystyrene beads. Adv Mater. 2000;12(3):206.
Yoon SB, Kim JY, Kim JH, Park YJ, Yoon KR, Park SK, Yu JS. Synthesis of monodisperse spherical silica particles with solid core and mesoporous shell: mesopore channels perpendicular to the surface. J Mater Chem. 2007;17:1758.
Lou XW, Yuan C, Archer LA. Shell-by-shell synthesis of tin oxide hollow colloids with nanoarchitectured walls: cavity size tuning and functionalization. Small. 2007;3(2):261.
Chen JS, Archer LA, Lou XW. SnO2 hollow structures and TiO2 nanosheets for lithium-ion batteries. J Mater Chem. 2011;21:9912.
Lou XW, Yuan C, Archer LA. Double-walled SnO2 nano-cocoons with movable magnetic cores. Adv Mater. 2007;19(20):3328.
Nguyen CC, Vu NN, Do TO. Efficient hollow double-shell photocatalysts for the degradation of organic pollutants under visible light and in darkness. J Mater Chem A. 2016;4:4413.
Joo JB, Kim P, Kim W, Kim J, Kim ND, Yi J. Simple preparation of hollow carbon sphere via templating method. Curr Appl Phys. 2008;8(6):814.
Hu Y, Ge J, Sun Y, Zhang T, Yin Y. A self-templated approach to TiO2 microcapsules. Nano Lett. 2007;7(6):1832.
Zhang Q, Lee I, Ge J, Zaera F, Yin Y. Surface-protected etching of mesoporous oxide shells for the stabilization of metal nanocatalysts. Adv Funct Mater. 2010;20(14):2201.
Pirzada T, Arvidson SA, Saquing CD, Shah SS, Khan SA. Hybrid carbon silica nanofibers through sol–gel electrospinning. Langmuir. 2014;30(51):15504.
Zhang T, Ge J, Hu Y, Zhang Q, Aloni S, Yin Y. Formation of hollow silica colloids through a spontaneous dissolution-regrowth process. Angew Chem Int Ed. 2008;47(31):5806.
Fang X, Chen C, Liu Z, Liu P, Zheng N. A cationic surfactant assisted selective etching strategy to hollow mesoporous silica spheres. Nanoscale. 2011;3:1632.
Yang HG, Zeng HC. Preparation of hollow anatase TiO2 nanospheres via ostwald ripening. J Phys Chem B. 2004;108(11):3492.
Li J, Zeng HC. Hollowing Sn-doped TiO2 nanospheres via ostwald ripening. J Am Chem Soc. 2007;129(51):15839.
Lou XW, Wang Y, Yuan C, Lee JY, Archer LA. Template-free synthesis of SnO2 hollow nanostructures with high lithium storage capacity. Adv Mater. 2006;18(17):2325.
Xu Y, Zhang Y, Zhou Y, Xiang S, Wang Q, Zhang C, Sheng X. CeO2 hollow nanospheres synthesized by a one pot template-free hydrothermal method and their application as catalyst support. RSC Adv. 2015;5:58237.
Cao CY, Cui ZM, Chen CQ, Song WG, Cai W. Ceria hollow nanospheres produced by a template-free microwave-assisted hydrothermal method for heavy metal ion removal and catalysis. J Phys Chem C. 2010;114(21):9865.
Chang Y, Teo JJ, Zeng HC. Formation of colloidal CuO nanocrystallites and their spherical aggregation and reductive transformation to hollow Cu2O nanospheres. Langmuir. 2004;21(3):1074.
Liu B, Zeng HC. Symmetric and asymmetric ostwald ripening in the fabrication of homogeneous core–shell semiconductors. Small. 2005;1(5):566.
Liu J, Qiao SZ, Liu H, Chen J, Orpe A, Zhao D, Lu GQ. Extension of the Stöber method to the preparation of monodisperse resorcinol-formaldehyde resin polymer and carbon spheres. Angew Chem Int Ed. 2011;50(26):5947.
Lee YJ, Joo JB, Yin Y, Zaera F. Evaluation of the effective photoexcitation distances in the photocatalytic production of H2 from water using Au@Void@TiO2 yolk–shell nanostructures. ACS Energy Lett. 2016;1(1):52.
Ge J, Zhang Q, Zhang T, Yin Y. Core-satellite nanocomposite catalysts protected by a porous silica shell: controllable reactivity, high stability, and magnetic recyclability. Angew Chem Int Ed. 2008;47(46):8924.
Dillon RJ, Joo JB, Zaera F, Yin Y, Bardeen CJ. Correlating the excited state relaxation dynamics as measured by photoluminescence and transient absorption with the photocatalytic activity of Au@TiO2 core–shell nanostructures. Phys Chem Chem Phys. 2013;15:1488.
Zhang Q, Shu XZ, Lucas JM, Toste FD, Somorjai GA, Alivisatos AP. Inorganic micelles as efficient and recyclable micellar catalysts. Nano Lett. 2014;14(1):379.
Kim M, Park JC, Kim A, Park KH, Song H. Porosity control of Pd@SiO2 yolk–shell nanocatalysts by the formation of nickel phyllosilicate and its influence on Suzuki coupling reactions. Langmuir. 2012;28(15):6441.
Nabid MR, Bide Y, Abuali M. Copper core silver shell nanoparticle–yolk/shell Fe3O4@chitosan-derived carbon nanoparticle composite as an efficient catalyst for catalytic epoxidation in water. RSC Adv. 2014;4:35844.
Li X, Zhang W, Zhang L, Yang H. Pd nanoparticles confined in fluoro-functionalized yolk–shell-structured silica for olefin hydrogenation in water. Chin J Catal. 2013;34(6):1192.
Acknowledgements
This work is supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry and Energy (MOTIE, No. 20174010201490). This work is also financially supported by the Korea Environment Industry & Technology Institute (KEITI) through “The Chemical Accident Prevention Technology Development Project” granted by the Korea Ministry of Environment (MOE, No. 2017001960004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, I., Lee, H.K., Lee, G.W. et al. Inorganic shell nanostructures to enhance performance and stability of metal nanoparticles in catalytic applications. Rare Met. 39, 767–783 (2020). https://doi.org/10.1007/s12598-019-01203-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-019-01203-8