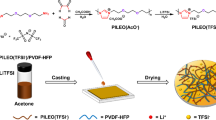
Abstract
High-performance solid polymer electrolyte (SPE) has long been desired for the next-generation high energy density and safe rechargeable lithium batteries. A SPE composed of 80 wt% lithium bis(trifluoromethanesulfonyl)imide (LiTFSI), 20% poly(ethylene carbonate) (PEC) and a polyamide (PA) fiber membrane backbone was prepared by solution-casting method. This solid electrolyte exhibits quite high ionic conductivity and lithium ion transference number (t+), and excellent mechanical strength. The as-prepared solid electrolyte shows good wettability to porous electrodes during cycles, which is beneficial to form ionically conductive phase throughout porous electrodes. All-solid-state LiFePO4|Li cells assembled with the as-prepared solid electrolyte deliver a high initial discharge specific capacity of 125.7 mAh·g−1 and good cycling stability at 55 °C (93.4% retention at 1C after 200 cycles), and superior cycle performance. Outstanding electrochemical performance can be mainly ascribed to the improved ionic conductivity in the entire porous electrodes due to the good wettability of SPE.






Similar content being viewed by others
References
Evarts EC. Lithium batteries: to the limits of lithium. Nature. 2015; 526(7575): S93-5.
Dunn B, Kamath H, Tarascon JM. Electrical energy storage for the grid: a battery of choices. Science. 2011;334(6058):928.
Wu H, Chan G, Choi JW, Ryu I, Yao Y, McDowell MT, Lee SW, Jackson A, Yang Y, Hu LB, Cui Y. Stable cycling of double-walled silicon nanotube battery anodes through solid-electrolyte interphase control. Nat Nanotechnol. 2012;7(5):310.
Lin DC, Lu ZD, Hsu PC, Lee HR, Liu N, Zhao J, Wang HT, Liu C, Cui Y. A high tap density secondary silicon particle anode fabricated by scalable mechanical pressing for lithium-ion batteries. Energy Environ Sci. 2015;8(8):2371.
Ding F, Xu W, Graff GL, Zhang J, Sushko ML, Chen XL, Shao YY, Engelhard MH, Nie ZM, Xiao J, Liu XJ, Sushko PV, Liu J, Zhang JG. Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J Am Chem Soc. 2013;135(11):4450.
Yan K, Lee HW, Gao T, Zheng GY, Yao HB, Wang HT, Lu ZD, Zhou Y, Liang Z, Liu ZF, Chu S, Cui Y. Ultrathin two-dimensional atomic crystals as stable interfacial layer for improvement of lithium metal anode. Nano Lett. 2014;14(10):6016.
Sun YK, Myung ST, Park BC, Prakash J, Belharouak I, Amine K. High-energy cathode material for long-life and safe lithium batteries. Nat Mater. 2009;8(4):320.
Deng LZ, Wu F, Gao XG, Wu WP. Development of a LiFePO4-based high power lithium secondary battery for HEVs applications. Rare Met. 2014. https://doi.org/10.1007/s12598-014-0316-1.
Su D, Cortie M, Fan H, Wang G. Prussian blue nanocubes with an open framework structure coated with PEDOT as high-capacity cathodes for lithium–sulfur batteries. Adv Mater. 2017;5(24):1501082.
Hao YC, Xiong DB, Liu W, Fan LL, Li DJ, Li XF. Controllably designed “vice-electrode” interlayers harvesting high performance lithium sulfur batteries. ACS Appl Mater Interfaces. 2017;9(46):40273.
Park K, Cho JH, Jang JH, Yu BC, Andreah T, Miller KM, Ellison CJ, Goodenough JB. Trapping lithium polysulfides of a Li–S battery by forming lithium bonds in a polymer matrix. Energy Environ Sci. 2015;8(8):2389.
Auvergniot J, Cassel A, Ledeuil JB, Viallet V, Seznec V, Dedryvère R. Interface stability of argyrodite Li6PS5Cl toward LiCoO2, LiNi1/3Co1/3Mn1/3O2, and LiMn2O4 in bulk all-solid-state batteries. Chem Mater. 2017;29(9):3883.
Lee S, Cho Y, Song HK, Lee KT, Cho J. Carbon-coated single-crystal LiMn2O4 nanoparticle clusters as cathode material for high-energy and high-power lithium-ion batteries. Angew Chem Int Ed. 2012;51(35):8748.
Liu XG, Tan J, Fu J, Yuan RX, Wen H, Zhang CH. Facile synthesis of nanosized Lithium-ion-conducting solid electrolyte Li1.4Al0.4Ti1.6(PO4)3 and its mechanical nanocomposites with LiMn2O4 for enhanced cyclic performance in lithium ion batteries. ACS Appl Mater Interfaces. 2017;9(13):11696.
Tao XY, Liu YY, Liu W, Zhou GM, Zhao J, Lin DC, Zu CX, Sheng OW, Zhang WK, Lee HW, Cui Y. Solid-state lithium–sulfur batteries operated at 37 °C with composites of nanostructured Li7La3Zr2O12/carbon foam and polymer. Nano Lett. 2017;17(5):2967.
Keller M, Appetecchi GB, Kim GT, Sharova V, Schneider M, Schuhmacher J, Roters A, Passerini S. Electrochemical performance of a solvent-free hybrid ceramic-polymer electrolyte based on Li7La3Zr2O12 in P(EO)15LiTFSI. J Power Sour. 2017;353(6):287.
Wang YF, Wang LX, Yuan QB, Chen J, Niu YJ, Xu XW, Cheng YT, Yao B, Wang Q, Wang H. Ultrahigh energy density and greatly enhanced discharged efficiency of sandwich-structured polymer nanocomposites with optimized spatial organization. Nano Energy. 2018;44(2):364.
Zeng XX, Yin YX, Li NW, Du WC, Guo YG, Wan LJ. Reshaping lithium plating/stripping behavior via bifunctional polymer electrolyte for room-temperature solid Li metal batteries. J Am Chem Soc. 2016;138(49):15825.
Wang QJ, Jian ZX, Song WL, Zhang SC, Fan LZ. Facile fabrication of safe and robust polyimide fibrous membrane based on triethylene glycol diacetate-2-propenoic acid butyl ester gel electrolytes for lithium-ion batteries. Electrochim Acta. 2014;149(12):176.
Fan LZ, Nan CW, Zhao SJ. Effect of modified SiO2 on properties of PEO-based polymer electrolytes. Solid State Ion. 2003;164(12):81.
Devaux D, Glé D, Phan TNT, Gigmes D, Giroud E, Deschamps M, Denoyel R, Bouchet R. Optimization of block copolymer electrolytes for lithium metal batteries. Chem Mater. 2015;27(13):4682.
Chen L, Liu YC, Fan LZ. Enhanced interface stability of polymer electrolytes using organic cage-type cucurbit [6] uril for lithium metal batteries. J Electrochem Soc. 2017;164(9):A1834.
Xu K. Electrolytes and interphases in Li-ion batteries and beyond. Chem Rev. 2014;114(23):11503.
Wang QJ, Song WL, Wang LN, Song Y, Shi Q, Fan LZ. Electrospun polyimide-based fiber membranes as polymer electrolytes for lithium-ion batteries. Electrochim Acta. 2014;132(6):538.
Wang QJ, Song WL, Fan LZ, Song Y. Flexible, high-voltage and free-standing composite polymer electrolyte membrane based on triethylene glycol diacetate-2-propenoic acid butyl ester copolymer for lithium-ion batteries. J Membr Sci. 2015;492(10):490.
Diederichsen KM, McShane EJ, McCloskey BD. Promising routes to a high Li+ transference number electrolyte for lithium ion batteries. ACS Energy Lett. 2017;2(11):2563.
Ghosh A, Wang C, Kofinas P. Block copolymer solid battery electrolyte with high Li-ion transference number. J Electrochem Soc. 2010;157(7):A846.
Xu K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem Rev. 2004;104(10):4303.
Tominaga Y, Yamazaki K. Fast Li-ion conduction in poly(ethylene carbonate)-based electrolytes and composites filled with TiO2 nanoparticles. Chem Commun. 2014;50(34):4448.
Okumura T, Nishimura S. Lithium ion conductive properties of aliphatic polycarbonate. Solid State Ion. 2014;267(12):68.
Kimura K, Matsumoto H, Hassoun J, Panero S, Scrosati B, Tominaga Y. A quaternarypoly(ethylene carbonate)-lithium bis(trifluoromethanesulfonyl) imide-ionic liquid-silica fiber composite polymer electrolyte for lithium batteries. Electrochim Acta. 2015;175(9):134.
Morioka T, Ota K, Tominaga Y. Effect of oxyethylene side chains on ion-conductive properties of polycarbonate-based electrolytes. Polymer. 2016;84(2):21.
Zhang JJ, Zhao JH, Yue LP, Wang QF, Chai JC, Liu ZH, Zhou XH, Li H, Guo YG, Cui GL, Chen LQ. Safety-reinforced poly(propylene carbonate)-based all-solid-state polymer electrolyte for ambient-temperature solid polymer lithium batteries. Adv Energy Mater. 2015;5(24):1501082.
Zhao JH, Zhang JJ, Hu P, Ma J, Wang XG, Yue LP, Xu GJ, Qin BS, Liu ZH, Zhou XH, Cui GL. A sustainable and rigid-flexible coupling cellulose-supported poly(propylene carbonate) polymer electrolyte towards 5 V high voltage lithium batteries. Electrochim Acta. 2016;188(1):23.
Sun B, Mindemark J, Edström K, Brandell D. Polycarbonate-based solid polymer electrolytes for Li-ion batteries. Solid State Ion. 2014;262(9):7–38.
Silva MM, Barros SC, Smith MJ, MacCallum JR. Characterization of solid polymer electrolytes based on poly(trimethylenecarbonate) and lithium tetrafluoroborate. Electrochim Acta. 2004;49(12):1887.
Shim J, Kim DG, Kim HJ, Lee JH, Baik JH, Lee JC. Novel composite polymer electrolytes containing poly(ethylene glycol)-grafted graphene oxide for all-solid-state lithium-ion battery applications. J Mater Chem A. 2014;2(34):13873.
Wang AL, Xu H, Zhou Q, Liu X, Li ZY, Gao R, Wu N, Guo YG, Li HY, Zhang LY. A new all-solid-state hyperbranched star polymer electrolyte for lithium ion batteries: synthesis and electrochemical properties. Electrochim Acta. 2016;212(9):372.
Lim SK, Setiawan L, Bae TH, Wang R. Polyamide-imide hollow fiber membranes crosslinked with amine-appended inorganic networks for application in solvent-resistant nanofiltration under low operating pressure. J Membr Sci. 2016;501(3):152.
Kong X, Zhou MY, Lin CE, Wang J, Zhao B, Wei XZ, Zhu BK. Polyamide/PVC based composite hollow fiber nanofiltration membranes: effect of substrate on properties and performance. J Membr Sci. 2016;505(5):231.
Evans J, Vincent CA, Bruce PG. Electrochemical measurement of transference numbers in polymer electrolytes. Polymer. 1987;28(13):2324.
Wu XL, Guo YG, Su J, Xiong JW, Zhang YL, Wan LJ. Carbon-nanotube-decorated nano-LiFePO4@C cathode material with superior high-rate and low-temperature performances for lithium-ion batteries. Adv Energy Mater. 2013;3(9):1155.
Zhong SK, Wu L, Liu JQ. Sol-gel synthesis and electrochemical properties of 9LiFePO4·Li3V2(PO4)3/C composite cathode material for lithium ion batteries. Electrochim Acta. 2012;74(7):8.
Wang QJ, Song WL, Fan LZ, Song Y. Facile fabrication of polyacrylonitrile/alumina composite membranes based on triethylene glycol diacetate-2-propenoic acid butyl ester gel polymer electrolytes for high-voltage lithium-ion batteries. J Membr Sci. 2015;486(7):21.
Sun B, Liao IY, Tan S, Bowden T, Brandell D. Solid polymer electrolyte coating from a bifunctional monomer for three-dimensional microbattery applications. J Power Sour. 2013;238(9):435.
Wetjen M, Navarra MA, Panero S, Passerini S, Scrosati B, Hassoun J. Composite poly(ethylene oxide) electrolytes plasticized by N-Alkyl-N-butylpyrrolidinium bis(trifluoromethanesulfonyl) imide for lithium batteries. Chemsuschem. 2013;6(6):1037.
Acknowledgments
This work was financially supported by the National Natural Scientific Foundation of China (No. 51532002), Beijing Natural Science Foundation (No. L172023), the National Basic Research Program of China (No. 2015CB932500).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, ZJ., Fan, LZ. Poly(ethylene carbonate)-based electrolytes with high concentration Li salt for all-solid-state lithium batteries. Rare Met. 37, 488–496 (2018). https://doi.org/10.1007/s12598-018-1017-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-018-1017-y