Abstract
We tested the hypothesis that there are sex differences in hindlimb unloading-induced activation of the forkhead box subfamily O3a (FoxO3a) signaling pathway in rat soleus muscle. Age-matched male and female Wistar rats were subjected to hindlimb unloading, and the soleus muscle was removed before or 1 or 7 days after unloading. Female rats showed greater percent changes in relative soleus muscle weight than males. FoxO3a phosphorylation was lower in females than in males and was associated with higher levels of protein ubiquitination 7 days after unloading. Heat shock protein 72 (Hsp72) levels were lower in female rats and increased in males during unloading. Female rats showed slightly higher myostatin levels, which showed a non-significant decline in male rats following unloading. Thus, males and females show different responses to the FoxO3a/ubiquitin–proteasome pathway following hindlimb unloading in rat soleus muscle, which may be associated with differences in Hsp72 expression and myostatin signaling.
Similar content being viewed by others
Introduction
Males and females exhibit marked physiological differences, including body composition [1], hormone concentration [2], muscle fiber type [3], and substrate utilization [4]. These differences can affect muscle response and adaptation to extracellular stimuli. For example, young and older men generally exhibit a greater hypertrophic response to resistance training [5, 6]. In contrast to this positive adaptation by men, women exhibit increased response to disuse [7, 8]—for instance, 17 weeks of horizontal bed rest induced a greater reduction in whole muscle volume in female as compared to male subjects [8]. Another study showed that recovery of muscle strength from unloading is slower for women than for men [9]. Thus, unloading-induced skeletal muscle loss may be sex-specific; however, there is limited supporting evidence for this possibility and the underlying mechanisms remain unclear.
Forkhead box subfamily (Fox)O transcription factors are involved in metabolism, apoptosis, and cell cycle progression in skeletal muscle [10]. FoxO signaling regulates two major catabolic systems—i.e., the ubiquitin–proteasome and autophagy–lysosome pathways, both of which mediate muscle protein degradation during skeletal muscle atrophy [11, 12]. Interestingly, women express higher levels of FoxO3 mRNA than men [13], suggesting that differential FoxO3 signaling in response to unloading is responsible for the higher degree of skeletal muscle loss during disuse in females. Moreover, its upstream molecules such as Akt, Hsp72, and myostatin also exhibit sex-specific abundance or response to several extracellular stimuli in human and rat skeletal muscle [14,15,16]; however, there have been no studies investigating changes in intracellular signaling associated with muscle loss.
To address this issue, in this study we examined sex differences in the activation of the FoxO3a/ubiquitin–proteasome pathway following hindlimb unloading in rat soleus muscle. Our results provide new insight into sex-specific changes in FoxO3 signaling under conditions of muscle atrophy.
Materials and methods
Experimental animals and hindlimb unloading
This study was approved by the Juntendo University Animal Care Committee (H28-15) and followed the principles for the care and use of laboratory animals set forth by the Physiological Society of Japan. Age-matched male and female Wistar rats (10 weeks old; n = 18/group) used in this study were housed in a climate-controlled room (23 ± 1 °C; 55 ± 5% relative humidity, and 12:12-h light/dark cycle) and had free access to standard rat chow and water. After acclimation, both male (255.1 ± 2.0 g) and female (164.2 ± 1.4 g) rats were subjected to hindlimb unloading for 1 or 7 days as previously described [17]. Briefly, a tail-cast suspension was applied to each rat, leaving the distal one-third of the tail free to allow proper thermoregulation. The tail cast was attached to a hook on the ceiling of the cage, and the height of the hook was adjusted so that the cast was inclined at an angle of ~ 35° in a head-down orientation. The rat was free to move around the cage on its front feet. Rats were checked daily for tail lesions or discoloration. After the experimental period, the rats were anesthetized with isoflurane (~ 3–5%) and pentobarbital sodium (60 mg/kg); the soleus muscle was carefully removed before (0 day; n = 6/group) or 1 day (n = 6/group) or 7 days (n = 6/group) after hindlimb unloading and weighed. Muscle weight per body weight was expressed as relative muscle weight. The muscles were flash frozen in liquid nitrogen and stored at − 80 °C until analysis.
Muscle preparation
Frozen soleus muscle tissue was powdered and a 30-mg sample was analyzed. Subcellular fractions were prepared from powdered muscles using a commercially available extraction kit (Thermo Fisher Scientific, Waltham, MA, USA). Briefly, ~ 30 mg of skeletal muscle was homogenized in CER-I buffer containing complete EDTA-free and PhosSTOP protease inhibitor cocktails (Roche, Penzberg, Germany) using a bead cell disrupter (Microsmash MS-100; Tomy Seiko Co., Tokyo, Japan). The lysate was centrifuged at 500 × g for 5 min at 4 °C and the pellet was washed three times in homogenization buffer to remove contaminating cytosolic proteins. The pellet was then resuspended in nuclear extraction reagent buffer supplemented with protease inhibitor according to the manufacturer’s instructions. To obtain the myofibril fraction, the insoluble pellet corresponding to the particulate fraction was washed three times in five volumes of ice-cold homogenization buffer and centrifuged at 12,000 × g for 5 min at 4 °C, then resuspended in ten volumes of lysis buffer [20 mM HEPES (pH 7.4); 250 mM NaCl, 1% (w/v) lithium dodecyl sulfate] and centrifuged at 17,000 × g for 5 min at 4 °C. To obtain the pure cytosolic fraction, the sample was centrifuged at 12,000 × g for 15 min at 4 °C and the supernatant was collected. Protein concentration was determined using a bicinchoninic acid protein assay kit (Thermo Fisher Scientific).
Immunodetection
Equal amounts of protein (20 μg) were loaded onto a precast 4–15% Tris–Glycine extended polyacrylamide gel (Bio-Rad, Copenhagen, Denmark) and electrophoretically separated at 150 V for 60 min. Separated proteins were transferred to a polyvinylidene difluoride membrane (Bio-Rad) that was incubated in blocking reagent (Can Get Signal; Toyobo, Tokyo, Japan). After three washes, the membrane was incubated with primary antibodies against the following proteins: β-actin (1:2000), phosphorylated Ser253-FoxO3a (1:1000), LC3A/B (1:2000), phosphorylated Ser465/467-Smad2/Ser423/425-Smad3 (1:2000), Smad2/Smad3 (1:2000), phosphorylated Ser473-Akt (1:2000), and Akt (1:2000) (all from Cell Signaling Technology, Beverly, MA, USA); FoxO3a (1:1000; Millipore, Temecula, CA, USA); mono- and polyubiquitinated conjugates (Enzo Therapeutics, Farmingdale, NY, USA); GDF-8/myostatin (1:2000; Abbiotec, San Diego, CA, USA); and follistatin (1:2000; Aviva Systems Biology, San Diego, CA, USA). This was followed by incubation with anti-rabbit horseradish peroxidase-conjugated secondary antibodies (1:5000; Cell Signaling Technology) in dilution buffer (Can Get Signal; Toyobo) for 1 h at room temperature (25–26 °C). After several washes, protein bands were visualized using enhanced chemiluminescence Prime reagent (GE Healthcare, Piscataway, NJ, USA), and the signal was recorded using a ChemiDoc Touch Imaging System (Bio-Rad). Signal intensity was analyzed using Image Lab v.5.2.1 software (Bio-Rad). Phosphorylation ratios were normalized to that of the male 0 day group (n = 6), which was set as 1.0. Immunodetection of β-actin or Ponceau red staining was used as a loading control.
To determine Hsp72 expression level, membranes were incubated with anti-Hsp72 alkaline phosphatase conjugate (1:2000; Stressgen, Victoria, BC, Canada) in Tris-buffered saline with Tween 20 along with 5% non-fat dry milk for 1 h at room temperature. Membranes were reacted with alkaline phosphatase substrate (Immun-Star; Bio-Rad) at room temperature. Analyses were performed using Image Lab v.5.2.1 software.
Real-time polymerase chain reaction
Total RNA was isolated as previously described [18]. RNA was purified using an RNeasy Mini kit (Qiagen, Valencia, CA, USA), and the concentration and purity (A260/280 and A260/230, respectively) were determined using QIAxpert (Qiagen). Reverse transcription was performed with 2 μg of total RNA using SuperScript VILO MasterMix (Invitrogen, Carlsbad, CA, USA). mRNA levels of HSPA1A (Hsp72Rn04224718_u1), TGF-β (Rn01504766_m1), Smad2 (Rn00569900_m1), and Smad3 (Rn00565331_m1) were quantified using a TaqMan gene expression assay (Applied Biosystems, Foster City, CA, USA) and were normalized to 18S mRNA levels. The 2−ΔΔCt method was used for data analysis [cycle threshold (Ct) = Ct (gene of interest) − Ct (reference gene)], where Ct indicates the fractional cycle number at which the amount of amplified target reaches a fixed threshold. Relative changes (ΔΔCt) in the expression level of the target gene were calculated by subtracting the ΔCt of the male control rat (0 day of unloading).
Measurement of soleus myofiber cross-sectional area
Serial cross-sections (10 μm thick) of frozen soleus muscle samples were obtained using a cryostat (CM3050S, Leica, Wetzlar, Germany) at − 20 °C, and standard hematoxylin and eosin staining was performed to measure total myofiber cross-sectional area (CSA), as previously described [19]. Sections were captured using a microscope (20×; BZ-8000; Keyence, Osaka, Japan). The CSA was determined using ImageJ software (NIH, Bethesda, MD, USA) by tracing the outlines of individual fibers. The mean fiber CSA was then calculated by dividing the total area by the total number of fibers and was expressed in μm2.
Statistical analysis
Values are expressed as mean ± standard error of the mean. Statistical significance was evaluated by two-way (sex × unloading) analysis of variance. Simple effects tests were performed when the interaction was significant. When significant main effects were found (without significant interaction), pairwise comparisons were performed where necessary using Sidak’s method. The unpaired Student’s t-test was used to compare variables between males and females. p < 0.05 was considered statistically significant. All analyses were performed using Prism v.6.0 software (GraphPad Inc., La Jolla, CA, USA).
Results
Body weight, soleus muscle weight, fiber CSA
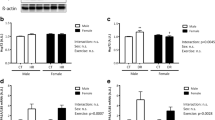
We measured the body weight, change and percent change in relative soleus muscle weight, and soleus muscle fiber CSA during hindlimb unloading (Fig. 1) and found that male and female rats showed similar decreases in body weight (Fig. 1a), soleus muscle weight (data not shown) and soleus muscle fiber CSA than males (Fig. 1c, d) in response to unloading. However, there were sex differences in the decrease in relative soleus muscle weight (Fig. 1b). Importantly, although the percent change in soleus muscle weight at 7 days did not differ significantly from the mean value on day 0 (males − 25.7% ± 2.8%; females − 28.0% ± 0.6%), females showed a greater change in relative soleus muscle weight and fiber CSA than males (− 14.5% ± 2.9% and − 30.0% ± 0.9% vs. − 24.4% ± 0.7% and − 39.6% ± 2.6%) (p < 0.01; Fig. 1e).
Body weight (a), relative soleus muscle weight (b), soleus muscle fiber CSA (c), hematoxylin and eosin staining of rat soleus muscle sections (magnification 20×, Scale bar = 50 μm) (d) and percent change in soleus muscle weight and fiber CSA (e) after 7 days of hindlimb unloading. SOL soleus, m. muscle, CSA cross-sectional area. Samples were collected before (0d), and 1 day (1d) and 7 days (7d) after hindlimb unloading. Values are mean ± standard error (SE); n = 6 per time point (CSA for 0d; n = 4). The results of two-way analysis of variance are displayed. **p < 0.01 vs. males
FoxO3a (Ser253) phosphorylation and ubiquitinated protein levels
Figure 2 shows the ratio of phosphorylated FoxO3a (Ser253), total FoxO3a expression level, and ubiquitinated protein level in the soleus muscle during hindlimb unloading. Total FoxO3a levels were unchanged during the experimental period in both males and females (Fig. 2a, c); however, FoxO3a phosphorylation was higher in males than in females 7 days after unloading (Fig. 2b). The main effects of differences in sex (p = 0.0006) and unloading time (p < 0.0001) were observed in ubiquitinated protein levels, which were higher in females than in males 7 days after unloading (Fig. 2d).
Representative blots (a), phosphorylation ratio of FoxO3a (Ser253), and total FoxO3a and ubiquitinated protein expression during 7 days of hindlimb unloading. Samples were collected before (0d), and 1 day (1d) and 7 days (7d) after hindlimb unloading. Equal protein loading was confirmed by Ponceau S staining. MW molecular weights (kDa). Values are mean ± SE; n = 6 per time point. The results of two-way analysis of variance are displayed. *p < 0.05 vs. males at each time point
Microtubule-associated protein 1A/1B light chain 3 (LC3) protein levels
The ratio of LC3II to LC3I protein—a marker for autophagy activation—during hindlimb unloading was evaluated (Fig. 3a, b). A significant change in this ratio was observed after unloading (p = 0.0129); however, it did not differ between the two sexes.
Representative blots (a) and the ratio of LC3 II to I expression (b) during 7 days of hindlimb unloading. Samples were collected before (0d), and 1 day (1d) and 7 days (7d) after hindlimb unloading. Values are mean ± SE; n = 6 per time point. β-Actin was used as a loading control. The results of two-way analysis of variance are displayed
Changes in Akt phosphorylation in response to muscle unloading
We examined the ratio of phosphorylated Akt (Ser473) to total Akt in soleus muscles during hindlimb unloading (Fig. 4a). There were significant differences between the sexes (p = 0.0182), and males showed higher Akt phosphorylation relative to females at 0 days after unloading (p < 0.0001; Fig. 4b). However, we found that the changes to hindlimb unloading were similar in both males and females (Fig. 4c).
Representative blots (a) and the phosphorylation ratio of Akt/PKB (Ser473) in (b) during 7 days of hindlimb unloading. Samples were collected before (0d), and 1 day (1d) and 7 days (7d) after hindlimb unloading. Values are mean ± SE; n = 6 per time point. The results of two-way analysis of variance are displayed. *p < 0.05 vs. males at each time point
Changes in heat shock protein (Hsp)72 mRNA and protein levels
Hsp72 mRNA and protein levels during hindlimb unloading were measured (Fig. 5a, c). Hsp72 protein levels changed significantly during unloading (p = 0.0002; Fig. 5b) and were higher in males than in females 7 days after unloading. Although the Hsp72 mRNA level also varied significantly (p < 0.0001), it did not differ between the sexes (Fig. 5c).
Representative blots (a), and Hsp72 protein (b) and HSPA1A mRNA (c) expression during 7 days of hindlimb unloading. Samples were collected before (0d), and 1 day (1d) and 7 days (7d) after hindlimb unloading. Values are mean ± SE; n = 6 per time point. β-Actin was used as a loading control and mRNA expression was normalized to that of 18S mRNA. The results of two-way analysis of variance are displayed
Changes in myostatin signal transducers and mRNA levels
We analyzed myostatin and follistatin protein levels; the mothers against decapentaplegic homolog (Smad)2 (Ser465/467) to Smad3 (Ser423/425) phosphorylation ratio; and transforming growth factor (TGF)-β, Smad2, and Smad3 mRNA levels in soleus muscle during hindlimb unloading (Fig. 6a, g). Myostatin protein levels differed significantly (p = 0.0282; Fig. 6a) and the level 7 days after unloading was higher than those on days 0 and 1 in females. Significant differences between sexes (p = 0.0046) and unloading times (p = 0.0002) were also observed in the Smad2 (Ser465/467) and Smad3 (Ser423/425) phosphorylation ratio, which was higher in males than in females 1 day after unloading (Fig. 6c). In contrast, no differences were observed in follistatin levels over the entire unloading period (Fig. 6b). Although TGF-β, Smad2, and Smad3 mRNA levels were altered after unloading (p < 0.0001), they did not differ between sexes (Fig. 6e–g).
Myostatin signaling transducers and mRNA expression. Myostatin (a), follistatin (b), phosphorylation ratio of Smad2 (Ser465/467)/Smad3 (Ser423/425) (c) and representative blots (d), and TGF-β (e), Smad2 (f) and Smad3 (g) mRNA expression. Samples were collected before (0d), and 1 day (1d) and 7 days (7d) after hindlimb unloading. Values are mean ± SE; n = 6 per time point. β-Actin was used as a loading control and mRNA expression was normalized to that of 18S mRNA. The results of two-way analysis of variance are displayed. *p < 0.05 vs. males at each time point
Discussion
The results of this study showed that 7 days of hindlimb unloading caused larger reductions in relative soleus muscle weight and fiber CSA in female as compared to male rats. Additionally, this is the first demonstration of sex differences in activation of the FoxO3a/ubiquitin–proteasome pathway following hindlimb unloading, which is a key regulator of muscle catabolism. Although the detailed mechanism remains to be elucidated, our findings imply that it involves downregulation of Hsp72 and increased myostatin signaling in the soleus muscle of female rats.
The main finding of our study was that hindlimb unloading reduced relative soleus muscle weight and fiber CSA in female rats, which was associated with upregulation of proteolytic markers such as dephosphorylated FoxO3a and myofibrillar protein ubiquitination. In the ubiquitin–proteasome pathway, proteins are targeted for degradation by the 26S proteasome, a large, multi-catalytic protease complex that degrades ubiquitinated proteins to small peptides by attaching ubiquitin molecules to target proteins to mark them for degradation [20]. Muscle protein degradation during disuse is mainly related to the ubiquitin–proteasome pathway [21, 22], and ubiquitination of myofibrillar proteins is a critical step in their degradation. Indeed, a previous study showed that soleus myofibrillar protein ubiquitination increased following short-duration hindlimb unloading (~ 8 days) and was correlated with decreases in muscle weight [23]. Moreover, FoxO3a plays a critical role in muscle atrophy, as it regulates the rate-limiting step of the ubiquitination process, which affects subsequent proteasome-dependent degradation. De-phosphorylation of FoxO3a induces its translocation into the nucleus, resulting in the upregulation of expression of muscle-specific ubiquitin ligase and autophagy-related genes, which are required for skeletal muscle metabolism. A previous study reported that the expression of dominant-negative FoxO3a inhibits immobilization-induced muscle atrophy, suggesting that the phosphorylation of FoxO3a is a key regulating factor during disuse-induced muscle wasting [24]. Thus, the inhibition of FoxO3a de-phosphorylation and subsequent protein ubiquitination steps are effective in attenuating disuse muscle atrophy [24, 25]. In the present study, we observed that the levels of ubiquitinated protein in the soleus muscle increased with unloading time in female rats. This suggests that these rats experienced increased breakdown of muscle components as compared to males during the early stage of unloading, resulting in a greater decrease in soleus muscle mass. However, we did not detect a sex difference in the level of LC3, a well-known autophagy marker regulated by FoxO3a; thus, induction of autophagy did not contribute to the difference in loss of muscle mass between female and male rats, despite the involvement of autophagy in controlling skeletal muscle protein turnover [26].
FoxO3a phosphorylation by Akt [27, 28] leads to its exclusion from the nucleus and inhibition of its transcriptional activity [28]. In the present study, although phosphorylated Akt level was higher in male rats than in female rats, the two sexes showed similar responses to unloading, which indicated a decline in protein synthesis during unloading. Thus, other upstream regulators are responsible for the sex differences in FoxO3a phosphorylation status and downstream protein ubiquitination. A previous study reported that Hsp72 overexpression directly inhibits FoxO3a activation (de-phosphorylation) at Ser253 in atrophied rat soleus muscle [25]. Hsp72 blocks FoxO3a nuclear localization during muscle atrophy, thereby contributing to the maintenance of phosphorylated FoxO3a levels [24, 25]. Although the precise contribution of Hsp72 to FoxO3a phosphorylation during unloading remains unclear, our results suggest that HSP responses differ in males and females during unloading. HSPs are stress-induced molecular chaperones that regulate correct protein folding and intracellular protein transport [29]. Many studies have shown that Hsp72 expression is upregulated in skeletal muscle following exercise [30, 31], muscle injury [32], and during muscle regrowth and regeneration [32, 33]; however, reports of Hsp72 levels during disuse are inconsistent, possibly due to the different animal models used and experimental periods analyzed. One study reported that 8 days of hindlimb unloading significantly decreased Hsp72 content in the soleus muscle of female Sprague–Dawley rats [34]. In contrast, others observed no changes in Hsp72 protein levels within 5–8 days of hindlimb unloading and immobilization in male Wistar and Sprague–Dawley rats [17, 35,36,37,38]. Indeed, numerous studies have shown that Hsp72 was downregulated in male rats [39,40,41,42,43,44] after long-term unloading (from 10 days to 9 weeks) whereas over the short term (5–8 days), female but not male animals tended to show reduced HSP levels [17, 34,35,36,37,38]. These data suggest a defense mechanism against intracellular stress in male rats over a relatively short period of unloading. The fact that HSPA1A (Hsp72) mRNA levels were upregulated during unloading in both sexes implies that they exhibit similar susceptibilities to stress but may differentially regulate Hsp72 protein at the translational and/or post-translational levels; however, this remains unconfirmed. Given that Hsp72 overexpression in skeletal muscle prevents hindlimb unloading- and immobilization-induced atrophy in rodents and improves structural and functional recovery from muscle atrophy [24, 45], elevated Hsp72 protein levels in male rats could positively regulate skeletal muscle mass through downregulation of FoxO3a-mediated protein degradation pathways.
Interestingly, we observed sex differences in myostatin signaling following hindlimb unloading: female rats showed slight increases in myostatin level, in contrast to the non-significant decline in male rats (although the levels of the myostatin antagonist follistatin were similar). Our data also showed that downstream Smad2 and Smad3 activation was greater in female rats. Myostatin is also known as growth differentiation factor (GDF)-8 and is a member of the TGF-β superfamily that negatively regulates muscle mass [46, 47]. Myostatin binding to activin type IIB receptor (ActRIIB) leads to Smad2/3 phosphorylation and activation and formation of a complex with Smad4 [48], which translocates to the nucleus to inhibit the transcription of genes involved in cell proliferation and differentiation and protein metabolism in skeletal muscle [49]. Moreover, activation of Smad2/3 impairs Akt activity, leading to inhibition of mechanistic target of rapamycin signaling-dependent protein synthesis and stimulation of FoxO3a-dependent protein degradation [46, 50]. The observed sex differences in response to unloading may be due to higher levels of ActRIIB mRNA in females [13]; however, the underlying mechanism remains to be determined. Recent evidence suggests that female subjects show greater increases in TGF-β signaling in response to acute resistance exercise [15]; thus, the increased response of myostatin signaling to various cellular stressors may negatively affect muscle mass maintenance during hindlimb unloading as well as following resistance training.
In conclusion, our data indicate that there are sex differences in the activation of the FoxO3a/ubiquitin–proteasome pathway following hindlimb unloading, which could be associated with distinct responses in Hsp72 protein regulation and myostatin signaling activation. However, our study was unable to reveal which factors are the primary triggers of the enhancement of FoxO3a signaling in female rats, as we did not consider the female estrous cycle, sex hormones (estrogen and testosterone), or other sex differences in hormone concentrations (such as glucocorticoid hormone) that may affect muscle protein turnover. Furthermore, FoxO3a signaling is regulated by a variety of external stimuli, such as insulin, insulin-like growth factor, nutrients, cytokines, and oxidative stress. Therefore, future studies are required to clarify the precise mechanisms of the sex-specific differences in FoxO3a signaling during hindlimb unloading.
References
Geer EB, Shen W (2009) Gender differences in insulin resistance, body composition, and energy balance. Gend Med 6(Suppl 1):60–75
Callewaert F, Sinnesael M, Gielen E, Boonen S, Vanderschueren D (2010) Skeletal sexual dimorphism: relative contribution of sex steroids, GH–IGF1, and mechanical loading. J Endocrinol 207(2):127–134
Haizlip KM, Harrison BC, Leinwand LA (2015) Sex-based differences in skeletal muscle kinetics and fiber-type composition. Physiology (Bethesda) 30(1):30–39
Roepstorff C, Steffensen CH, Madsen M, Stallknecht B, Kanstrup IL, Richter EA, Kiens B (2002) Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. Am J Physiol Endocrinol Metab 282(2):E435–E447
Hubal MJ, Gordish-Dressman H, Thompson PD, Price TB, Hoffman EP, Angelopoulos TJ, Gordon PM, Moyna NM, Pescatello LS, Visich PS, Zoeller RF, Seip RL, Clarkson PM (2005) Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc 37(6):964–972
Ivey FM, Roth SM, Ferrell RE, Tracy BL, Lemmer JT, Hurlbut DE, Martel GF, Siegel EL, Fozard JL, Jeffrey Metter E, Fleg JL, Hurley BF (2000) Effects of age, gender, and myostatin genotype on the hypertrophic response to heavy resistance strength training. J Gerontol Ser A: Biol Sci Med Sci 55(11):M641–M648
Ploutz-Snyder L, Bloomfield S, Smith SM, Hunter SK, Templeton K, Bemben D (2014) Effects of sex and gender on adaptation to space: musculoskeletal health. J Women’s Health (Larchmt) 23(11):963–966
Shackelford LC, LeBlanc AD, Driscoll TB, Evans HJ, Rianon NJ, Smith SM, Spector E, Feeback DL, Lai D (2004) Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol 97(1):119–129
Clark BC, Manini TM, Hoffman RL, Russ DW (2009) Restoration of voluntary muscle strength after 3 weeks of cast immobilization is suppressed in women compared with men. Arch Phys Med Rehabil 90(1):178–180
Kandarian SC, Jackman RW (2006) Intracellular signaling during skeletal muscle atrophy. Muscle Nerve 33(2):155–165
Tran H, Brunet A, Griffith EC, Greenberg ME (2003) The many forks in FOXO’s road. Sci STKE 2003(172):RE5
Mammucari C, Schiaffino S, Sandri M (2008) Downstream of Akt: FoxO3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy 4(4):524–526
Welle S, Tawil R, Thornton CA (2008) Sex-related differences in gene expression in human skeletal muscle. PLoS One 3(1):e1385
West DW, Burd NA, Churchward-Venne TA, Camera DM, Mitchell CJ, Baker SK, Hawley JA, Coffey VG, Phillips SM (2012) Sex-based comparisons of myofibrillar protein synthesis after resistance exercise in the fed state. J Appl Physiol 112(11):1805–1813
Liu D, Sartor MA, Nader GA, Gutmann L, Treutelaar MK, Pistilli EE, Iglayreger HB, Burant CF, Hoffman EP, Gordon PM (2010) Skeletal muscle gene expression in response to resistance exercise: sex specific regulation. BMC Genom 24(11):659
Voss MR, Stallone JN, Li M, Cornelussen RN, Knuefermann P, Knowlton AA (2003) Gender differences in the expression of heat shock proteins: the effect of estrogen. Am J Physiol Heart Circ Physiol 285(2):H687–H692
Yoshihara T, Sugiura T, Yamamoto Y, Shibaguchi T, Kakigi R, Naito H (2015) The response of apoptotic and proteolytic systems to repeated heat stress in atrophied rat skeletal muscle. Physiol Rep 3(10):e12597
Yoshihara T, Machida S, Kurosaka Y, Kakigi R, Sugiura T, Naito H (2016) Immobilization induces nuclear accumulation of HDAC4 in rat skeletal muscle. J Physiol Sci 66(4):337–343
Ichinoseki-Sekine N, Yoshihara T, Kakigi R, Sugiura T, Powers SK, Naito H (2014) Heat stress protects against mechanical ventilation-induced diaphragmatic atrophy. J Appl Physiol 117(5):518–524
Lecker SH, Goldberg AL, Mitch WE (2006) Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol 17(7):1807–1819
Furuno K, Goodman MN, Goldberg AL (1990) Role of different proteolytic systems in the degradation of muscle proteins during denervation atrophy. J Biol Chem 265(15):8550–8557
Tiao G, Fagan JM, Samuels N, James JH, Hudson K, Lieberman M, Fischer JE, Hasselgren PO (1994) Sepsis stimulates nonlysosomal, energy-dependent proteolysis and increases ubiquitin mRNA levels in rat skeletal muscle. J Clin Investig 94(6):2255–2264
Vermaelen M, Marini JF, Chopard A, Benyamin Y, Mercier J, Astier C (2005) Ubiquitin targeting of rat muscle proteins during short periods of unloading. Acta Physiol Scand 185(1):33–40
Senf SM, Dodd SL, McClung JM, Judge AR (2008) Hsp70 overexpression inhibits NF-kB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. FASEB J 22(11):3836–3845
Senf SM, Dodd SL, Judge AR (2010) FOXO signaling is required for disuse muscle atrophy and is directly regulated by Hsp70. Am J Physiol Cell Physiol 298(1):C38–C45
Sandri M (2013) Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. Int J Biochem Cell Biol 45(10):2121–2129
Yang W, Dolloff NG, El-Deiry WS (2008) ERK and MDM2 prey on FOXO3a. Nat Cell Biol 10(2):125–126
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96(6):857–868
Locke M (1997) The cellular stress response to exercise: role of stress proteins. Exerc Sport Sci Rev 25:105–136
Hernando R, Manso R (1997) Muscle fibre stress in response to exercise: synthesis, accumulation and isoform transitions of 70-kDa heat-shock proteins. Eur J Biochem 243(1–2):460–467
Milne KJ, Noble EG (2002) Exercise-induced elevation of HSP70 is intensity dependent. J Appl Physiol 93(2):561–568
Senf SM, Howard TM, Ahn B, Ferreira LF, Judge AR (2013) Loss of the inducible Hsp70 delays the inflammatory response to skeletal muscle injury and severely impairs muscle regeneration. PLoS One 8(4):e62687
Selsby JT, Rother S, Tsuda S, Pracash O, Quindry J, Dodd SL (2007) Intermittent hyperthermia enhances skeletal muscle regrowth and attenuates oxidative damage following reloading. J Appl Physiol 102(4):1702–1707
Naito H, Powers SK, Demirel HA, Sugiura T, Dodd SL, Aoki J (2000) Heat stress attenuates skeletal muscle atrophy in hindlimb-unweighted rats. J Appl Physiol 88(1):359–363
Selsby JT, Dodd SL (2005) Heat treatment reduces oxidative stress and protects muscle mass during immobilization. Am J Physiol Regul Integr Comp Physiol 289(1):R134–R139
Sugiura T, Ito N, Goto K, Naito H, Yoshioka T, Powers SK (2006) Estrogen administration attenuates immobilization-induced skeletal muscle atrophy in male rats. J Physiol Sci 56(6):393–399
Oishi Y, Ishihara A, Talmadge RJ, Ohira Y, Taniguchi K, Matsumoto H, Roy RR, Edgerton VR (2001) Expression of heat shock protein 72 in atrophied rat skeletal muscles. Acta Physiol Scand 172(2):123–130
Yoshihara T, Yamamoto Y, Shibaguchi T, Miyaji N, Kakigi R, Naito H, Goto K, Ohmori D, Yoshioka T, Sugiura T (2017) Dietary astaxanthin supplementation attenuates disuse-induced muscle atrophy and myonuclear apoptosis in the rat soleus muscle. J Physiol Sci 67(1):181–190
Takeda I, Fujino H, Murakami S, Kondo H, Nagatomo F, Ishihara A (2009) Thermal preconditioning prevents fiber type transformation of the unloading induced-atrophied muscle in rats. J Muscle Res Cell Motil 30(3–4):145–152
Fujino H, Ishihara A, Murakami S, Yasuhara T, Kondo H, Mohri S, Takeda I, Roy RR (2009) Protective effects of exercise preconditioning on hindlimb unloading-induced atrophy of rat soleus muscle. Acta Physiol (Oxf) 197(1):65–74
Gwag T, Lee K, Ju H, Shin H, Lee JW, Choi I (2009) Stress and signaling responses of rat skeletal muscle to brief endurance exercise during hindlimb unloading: a catch-up process for atrophied muscle. Cell Physiol Biochem 24(5–6):537–546
Lawler JM, Song W, Kwak HB (2006) Differential response of heat shock proteins to hindlimb unloading and reloading in the soleus. Muscle Nerve 33(2):200–207
Oishi Y, Ogata T, Yamamoto KI, Terada M, Ohira T, Ohira Y, Taniguchi K, Roy RR (2008) Cellular adaptations in soleus muscle during recovery after hindlimb unloading. Acta Physiol (Oxf) 192(3):381–395
Oishi Y, Taniguchi K, Matsumoto H, Kawano F, Ishihara A, Ohira Y (2003) Upregulation of HSP72 in reloading rat soleus muscle after prolonged hindlimb unloading. Jpn J Physiol 53(4):281–286
Miyabara EH, Nascimento TL, Rodrigues DC, Moriscot AS, Davila WF, AitMou Y, deTombe PP, Mestril R (2012) Overexpression of inducible 70-kDa heat shock protein in mouse improves structural and functional recovery of skeletal muscles from atrophy. Pflugers Arch 463(5):733–741
Elkina Y, von Haehling S, Anker SD, Springer J (2011) The role of myostatin in muscle wasting: an overview. J Cachexia Sarcopenia Muscle 2(3):143–151
McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 387(6628):83–90
Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L (2003) Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol Cell Biol 23(20):7230–7242
Allen DL, Unterman TG (2007) Regulation of myostatin expression and myoblast differentiation by FoxO and SMAD transcription factors. Am J Physiol Cell Physiol 292(1):C188–C199
Breitbart A, Auger-Messier M, Molkentin JD, Heineke J (2011) Myostatin from the heart: local and systemic actions in cardiac failure and muscle wasting. Am J Physiol Heart Circ Physiol 300(6):H1973–H1982
Acknowledgements
This work was supported by the Institute for Environmental and Gender-Specific Medicine (to T. Yoshihara), and by grants from the Japanese Center for Research on Women in Sports, Japan Society for the Promotion of Science KAKENHI (Grant no. 17K01765 to T. Yoshihara), and Ministry of Education, Culture, Sports, Science and Technology Supported Program for the Strategic Research Foundation at Private Universities.
Author information
Authors and Affiliations
Contributions
TY, RK, and HN conceived and designed the research; TY, TN, TT, and SC performed the experiments; TY and TN analyzed the data; TY and RK interpreted results of the experiments; TY prepared the figures; TY drafted the manuscript; TY, TN, TT, SC, RK, TS, and HN edited and revised the manuscript; TY, TN, TT, SC, RK, TS, and HN approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
About this article
Cite this article
Yoshihara, T., Natsume, T., Tsuzuki, T. et al. Sex differences in forkhead box O3a signaling response to hindlimb unloading in rat soleus muscle. J Physiol Sci 69, 235–244 (2019). https://doi.org/10.1007/s12576-018-0640-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12576-018-0640-6