Abstract
Osteoporosis and derangement of calcium homeostasis are common complications of thalassemia. Despite being an important process for bone and calcium metabolism, little is known about intestinal calcium transport in thalassemia. Recent reports of decreases in both intestinal calcium transport and bone mineral density in thalassemic patients and animal models suggested that defective calcium absorption might be a cause of thalassemic bone disorder. Herein, the possible mechanisms associated with intestinal calcium malabsorption in thalassemia are discussed. This includes alterations in the calcium transporters and hormonal controls of the transcellular and paracellular intestinal transport systems in thalassemia. In addition, the effects of iron overload on intestinal calcium absorption, and the reciprocal interaction between iron and calcium transport in thalassemia are elaborated. Understanding the mechanisms underlining calcium malabsorption in thalassemia would lead to development of therapeutic agents and mineral supplements that restore calcium absorption as well as prevent osteoporosis in thalassemic patients.
Similar content being viewed by others
Introduction
Calcium is one of the most important minerals in the body since it plays a crucial role in many physiological processes such as muscle contraction, neurotransmission, inflammation, blood clotting, intracellular signaling, and lactation. Maintenance of calcium homeostasis involves hormonal regulation of intestinal calcium absorption, bone remodeling process, and renal calcium excretion. Since calcium is obtained only through intestinal absorption, the amount of calcium absorbed partly determines the serum level of calcium, and consequently, bone mineral content and density. Calcium absorption is under the control of classical calciotropic hormones, i.e., parathyroid hormone (PTH) and 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], as well as some other humoral factors, such as calcitonin, prolactin, growth hormone, estrogen, and fibroblast growth factor (FGF)-23 [1,2,3,4,5]. A decrease in calcium absorption over a period of time can lead to a low level of serum calcium, and subsequently bone defects, which has been reported in many conditions and diseases including thalassemia.
Thalassemia is an inherited disease with hypochromic and microcytic anemia from defective α- or β-globin production [6]. It affects approximately four out of every 10,000 people globally [7], and more than 50% of β-thalassemic patients develop osteoporosis and osteopenia as well as bone deformity [8]. Other major problems in thalassemic patients and mutant animals consist of iron overload, splenomegaly, abnormal heart rhythm, diabetes mellitus, and growth retardation (for review, please see Nienhuis and Nathan [9]). A significant decrease in intestinal calcium transport has also been reported in thalassemic patients [10]. Severely impaired intestinal calcium absorption has also been observed in thalassemic mice, and was found to be associated with their lower bone mineral density (BMD) [11, 12]. Although the improvement of calcium absorption has been shown to be an effective way of preventing and relieving osteoporosis [13, 14], it is not known how calcium absorption is affected by thalassemic condition, and whether it could be the cause of bone defect. This review will discuss changes in the intestinal calcium-transport mechanisms and possible cause of intestinal calcium malabsorption in thalassemia with evidence from both human and animal studies.
Calcium transport and its hormonal control in healthy individuals
In human and other mammals, calcium enters the body mainly through ingestion. Dietary calcium is absorbed across the intestinal epithelial cells by two pathways, i.e., transcellular and paracellular pathways. Although both calcium-transport mechanisms take place along the entire length of the small intestine, the transcellular calcium transport is predominant in the proximal part, particularly the duodenum. Free-ionized calcium diffuses across the apical plasma membrane via transient receptor potential vanilloid calcium channel (TRPV) 5 and 6 and L-type voltage-dependent calcium channel (e.g., Cav1.3) [15]. Cytoplasmic calcium is then translocated by binding to calbindin-D9k and probably also to calbindin-D28k, parvalbumin, and calmodulin, to be extruded at the basolateral membrane through plasma membrane Ca2+-ATPase (PMCA) subtype 1b and Na+/Ca2+-exchanger (NCX)-1 [16,17,18]. Some intracellular vesicles can help ferry intracellular calcium and certain ions (e.g., iron) from the apical side to the basolateral side for extrusion [5].
Regarding the paracellular pathway, transepithelial transport of calcium occurs through space between neighboring two epithelial cells. Calcium movement is driven by the free energy of electrochemical gradient (passive diffusion) or by solvent drag [15, 19, 20]. For solvent drag-induced calcium transport, the basolateral Na+/K+-ATPase (NKA) pumps sodium into the paracellular space creating Na+-rich hyperosmotic microenvironment that, in turn, draws water from lumen to the plasma side simultaneously with ionized calcium [21, 22]. Paracellular calcium diffusion is indeed regulated by tight junction proteins, such as claudins, which possess size- and charge-selective properties [15, 19]. The expression of some claudins, particularly claudin-2 and -12, is dependent on 1,25(OH)2D3 and may be responsible for the 1,25(OH)2D3-induced calcium transport across the paracellular pathway [23]. Generally, under normal diets, the level of ionized calcium in the duodenal lumen is relatively high (~ 5 mmol/l) as compared with plasma ionized calcium (1.1–1.3 mmol/l) [19]. Thus, there is a calcium gradient across the duodenal epithelium, which can be a driving force for the paracellular calcium absorption. Our previous study has shown that luminal calcium concentration of ~ 5 mmol/l is substantial to induce the paracellular transport of calcium across the intestinal epithelium, and it can contribute up to 80% of the total calcium absorption, especially in the distal small intestine [19]. Moreover, the impaired paracellular pathway might also reduce the intestinal absorption of some other minerals, such as magnesium [24].
The relatively constant levels of plasma calcium are regulated by an integrative response of the calcium-regulating organs organized as the parathyroid–kidney–intestinal axis [25, 26]. For instance, decreases in plasma calcium stimulate the parathyroid gland to secrete PTH, which raises the plasma calcium level by (i) enhancing calcium reabsorption in the thick ascending limb of the Henle’s loop and distal renal tubule within minutes, (ii) stimulating bone resorption within minutes to hours, and (iii) stimulating 1α-hydroxylase in the proximal renal tubule to increase the production of 1,25(OH)2D3, which, in turn, potently stimulates intestinal calcium absorption within 24 h. PTH has also been reported to directly stimulate intestinal calcium absorption via L-type voltage-dependent calcium channel [27, 28]. On the other hand, an increase in plasma free-ionized calcium then induces negative feedback to inhibit PTH secretion through calcium-sensing receptor (CaSR), followed by a decrease in 1,25(OH)2D3 production, thereby reducing calcium absorption [29, 30]. Moreover, certain local and systemic humoral factors, e.g., FGF-23, may negatively regulate the duodenal calcium transport in a calcium and/or 1,25(OH)2D3-dependent manner.
FGF-23 was originally known as osteocyte/osteoblast-derived phosphatonin—i.e., a phosphorus-regulating hormone—and has recently been recognized as a new calcium-regulating hormone [31,32,33]. Khuituan et al. [31, 32] demonstrated a novel role of FGF-23 as a negative feedback regulator of the 1,25(OH)2D3-enhanced duodenal transcellular and paracellular calcium absorption in rodents. This finding provides an alternative explanation of how the duodenal enterocytes restrict excessive calcium transport and thus prevent lethal hypercalcemia. Meanwhile, an increase in serum phosphate level induces PTH and FGF-23 release that enhance phosphate excretion by suppressing 1α-hydroxylase and 1,25(OH)2D3 production [34, 35]. Calcium is also a potent stimulator of FGF-23 production via a vitamin D receptor (VDR)-independent manner [36].
Evidence of calcium malabsorption and osteoporosis in thalassemia
Osteoporosis and osteopenia are among the most common complications in thalassemia, and are found in > 50% of β-thalassemic patients, especially patients with thalassemia major, the most severe form of β-thalassemia caused by βo/βo genotype (i.e., no β-globin chain and no hemoglobin A) [8, 37, 38]. A study in prepubertal children (age 8–9 years old) showed a significant decrease in bone mineral density (BMD) in thalassemia major patients as compared to the age- and sex-matched constitutional short statue control [39]. Similarly, studies in children and adolescents (age 8–25 years old) with both transfusion-dependent and transfusion-independent β-thalassemia major showed that most patients experienced osteopenia/osteoporosis, bone pain and short stature related to impaired bone formation and growth compared to the age-matched controls [40,41,42,43]. The prevalence of bone fracture was approximately 12% in all types of thalassemic patients, including β-thalassemia major, β-thalassemia intermediate, thalassemia E/β and α-thalassemia, with an equal distribution between both sexes [44]. Another study covering patients with a variety of thalassemia genotypes and age range (6–75 years old) showed a high incidence of bone fracture, bone pain, and increased bone turnover that were correlated with decreased BMD [45]. Studies in animal models confirmed a high incidence of osteopenia/osteoporosis in thalassemia. Specifically, data from our studies using mice with C → T mutation at nucleotide 654 of intron 2 (βIVSII-654) and hemizygous knockout of β-globin gene (BKO) as β-thalassemic animal models showed that both hemizygous βIVSII-654 knockin and BKO mice manifested a significant reduction in BMD, bone mineral content (BMC), bone volume, and bone thickness, as compared to the wild-type controls [46,47,48].
A potential cause of decreased BMD leading to osteopenia/osteoporosis could result from an imbalance in bone remodeling process, i.e., elevated bone resorption and/or reduced bone formation. Bone histomorphometric analysis in thalassemic mice revealed that osteoclast surface, eroded surface, and osteoclast function were elevated in both hemizygous βIVSII-654 knockin and BKO thalassemic mice [46, 48]. Consistent with high bone resorption, higher circulating levels of osteoclastogenic factors including interleukin (IL)-1α, IL-1β, receptor activator of nuclear factor-κB ligand (RANKL), and tumor necrosis factor (TNF)-α were reported in thalassemic animals and patients, and were well associated with their decreased BMD [8, 47, 49,50,51]. These osteoclastogenic cytokines could also suppress osteoblast differentiation and activity, which in turn decrease bone formation. Furthermore, the known osteogenic factor, insulin-like growth (IGF)-1, was decreased in the serum of β-thalassemic patients together with lower BMD [39, 41, 42, 52, 53]. In contrast, serum levels of osteoblast differentiation inhibitors, namely Dickkopf-1 (a negative regulator of Wnt signaling) and sclerostin, were significantly higher in thalassemic patients [54, 55].
Other than the imbalanced bone remodeling process, hypocalcemia—possibly due to a decrease in calcium absorption—has been reported in both thalassemic animals and patients. Significant decreases in serum calcium and intestinal calcium absorption were observed in patients with thalassemia major together with the lower BMD [10, 43, 56, 57]. Evidence from animal studies, such as hemizygous βIVSII-654 knockin and BKO thalassemic mice, showed a marked decrease in calcium absorption across the small intestinal epithelium [11, 12, 58]. The phenomenon was present in both sexes of animals, and the daily injection of 1 µg/kg 1,25(OH)2D3 failed to restore normal intestinal calcium absorption in thalassemic mice despite being effective in enhancing calcium absorption in wild-type mice—presumably a sign of 1,25(OH)2D3 resistance [11]. Our recent study also found that thalassemia-induced calcium malabsorption could be rescued by a long-time treatment with lower dose of 1,25(OH)2D3 or treatment with hepcidin [12], which will be discussed further in the following section.
The alterations of the intestinal calcium-transport mechanisms in thalassemia
Direct evidence of thalassemia-induced changes in calcium transporters and/or the related proteins involved in intestinal calcium absorption is still limited. A study from our group showed downregulation of the transcellular calcium transporters and calcium transport-related proteins, i.e., TRPV5, TRPV6, calbindin-D9k, and PMCA1b, in thalassemic mice (Fig. 1) [11]. This impairment probably accounted for the reduction in intestinal calcium absorption. Besides these calcium transporters, other membrane-transporting proteins may be indirectly involved in calcium transport. The study in pernicious anemia showed the decreased activity of jejunal NKA, which is important for stabilization of the intracellular Na+ necessary for the extrusion of absorbed calcium via the basolateral NCX1 [59]. Furthermore, calcium-binding proteins, which take part in the cytoplasmic calcium translocation, also play an important role in intestinal calcium transport. In addition to our report of decrease in the intestinal expression of calbindin-D9k in thalassemic mice [11], there was another report of early life iron deficiency anemia that induced a decrease in calcium-binding protein parvalbumin in the rat hippocampus [60]. Even though the reported decreased parvalbumin in iron deficiency anemia came from a different organ system, it suggested a possibility of anemia-induced downregulation of calcium-binding protein expression in thalassemia. Taken together, the reduced levels of calcium transporters and related proteins could account for the impaired intestinal calcium transport as can be seen in thalassemic patients and animal models.
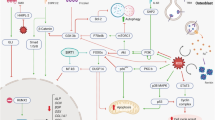
Schematic diagram shows possible mechanisms of calcium regulatory pathway and intestinal calcium absorption in thalassemia. Reduction of calcium-regulating hormones, i.e., parathyroid hormone (PTH), calcitonin, and 1,25(OH)2D3, in thalassemia leads to a decrease in the intestinal calcium absorption. Inset The decreased intestinal calcium absorption in thalassemia is due to downregulation of transcellular calcium transport-related proteins (TRPV5/6, PMCA1b, and NCX1). The possible mechanism may also involve downregulation of NKA and tight junction proteins, such as claudins, thereby compromising the paracellular calcium transport (for details, please see text). PVALB, parvalbumin; CaBP, calcium-binding proteins (e.g., calbindin-D9k); Cav1.3, voltage-dependent calcium channel 1.3
Paracellular intestinal calcium transport, another calcium transport system, represents the selective transportation of calcium through tight junction proteins, particularly claudins and occludin [5]. As mentioned earlier, 1,25(OH)2D3 has been shown to upregulate claudin-2 and -12, leading to the enhanced paracellular calcium transport in vitro and in vivo [5, 23]. Interestingly, hypoxia resulting from chronic anemia could lead to the activation of hypoxia-inducible factor (HIF)-1, which was also upregulated in the placentae of women with iron deficiency anemia and β-thalassemia trait carriers [61,62,63], and has been shown to suppress the expression of occludin and claudin-1 in human intestinal cells and rat duodenum [64, 65]. This body of evidence strongly supports the negative effect of thalassemia on the intestinal calcium transport (Fig. 1). However, more studies are needed to further provide the detailed molecular mechanisms of intestinal calcium transport impairment in thalassemia.
Thalassemia-induced calciotropic endocrinopathies
As shown in Fig. 1, hormonal control plays an important role in the regulation of intestinal calcium absorption. Therefore, the defects or abnormalities of these hormones in many diseases, including osteoporosis and thalassemia, could greatly affect intestinal calcium transport. One of the key regulators for intestinal calcium absorption is 1,25(OH)2D3, which directly stimulates calcium absorption by upregulating the expression and activities of several calcium transporters, e.g., TRPV5, TRPV6, calbindin-D9k [2, 31, 66]. PMCA1b expression and activity were also upregulated by 1,25(OH)2D3 in a human intestinal cell line and vitamin D-deficient mice, respectively [2, 67]. Furthermore, 1,25(OH)2D3 enhanced NCX activity in chick duodenum and NCX expression in duodenum of vitamin D-replete mice [31, 32, 68]. Taken together, it is clear that 1,25(OH)2D3 has a crucial role in intestinal calcium transport; therefore, decreases in 1,25(OH)2D3 level and/or its receptor can negatively affect the intestinal calcium transport.
In β-thalassemic patients, the level of serum 25-hydroxyvitamin D [25(OH)D] was significantly reduced as compared to the healthy individuals [42, 43, 69,70,71]. Impaired 1,25(OH)2D3 synthesis was also reported in β-thalassemia major patients [72]. In βIVSII−654 knockin thalassemic mice, vitamin D receptor was downregulated in the duodenal epithelial cells as compared to their wild-type littermates [11]. Other than thalassemia, patients with sickle cell anemia also showed a decreased serum vitamin D level [73]. The reduced serum 1,25(OH)2D3 in thalassemic patients and animals was correlated with low serum calcium levels and BMD, indicating that an impaired 1,25(OH)2D3 production and function could potentially cause intestinal calcium malabsorption in thalassemia.
PTH has been known to increase serum calcium level by reducing urinary calcium reabsorption, stimulating bone resorption, and indirectly enhancing intestinal calcium absorption by stimulating 1,25(OH)2D3 production. However, Picotto and coworkers showed that PTH might directly stimulate intestinal calcium transport, which was inhibited by Cav inhibitor [27]. Many studies reported the decreased serum PTH or hypoparathyroidism in patients with thalassemia major regardless of their age or blood transfusion status [40,41,42,43, 56, 69, 72, 74, 75]. Specifically, a reduction in serum PTH level is probably due to thalassemia-induced iron overload and iron deposit in the parathyroid gland, thus leading to parathyroid chief cell dysfunction and impairment of calcium homeostasis [76,77,78]. Decreased PTH was shown to correlate with lower serum and urine calcium levels and lower BMD in thalassemia [40, 43, 56, 57, 75]. Nevertheless, there was a paradox that low levels of PTH with its well-known bone resorption-stimulating activity were present with lower BMD. Taken together, thalassemic patients demonstrated reductions in serum calcium level and BMD that were associated with decreased PTH level, suggesting a possibility of intestinal calcium malabsorption from hypoparathyroidism in thalassemia. Furthermore, since PTH is a potent stimulator of renal 1,25(OH)2D3 production, the thalassemia-induced reduction in PTH level may cause a lower serum 1,25(OH)2D3, thereby reducing intestinal calcium transporter expression and transcellular calcium absorption [5, 79, 80]. A decrease in PTH level may directly aggravate calcium malabsorption in thalassemia because it can exert a direct stimulatory effect on the intestine by increasing cellular calcium uptake and extrusion [27, 28, 81].
Another calcium-regulating hormone, calcitonin, was demonstrated to negatively regulate intestinal calcium absorption in several studies [82, 83]. However, some studies found positive effects of calcitonin on intestinal calcium absorption. Specifically high-dose calcitonin could induce intestinal calcium absorption [84], and chronic calcitonin treatment was able to increase serum calcium level through the stimulation of 1,25(OH)2D3 production in rats [3, 85]. β-thalassemic patients were reported to have decreased levels of calcitonin and chronic calcitonin treatment could improve osteoporosis in these patients [52, 86], presumably due to an inhibitory effect of calcitonin on osteoclast function. Accordingly, the inappropriately decreased calcitonin levels in thalassemic patients could also contribute to intestinal calcium malabsorption possibly from lower 1,25(OH)2D3 production. Moreover, thalassemia-induced iron overload and iron deposit in the gonads further impaired production of sex steroids, particularly 17β-estradiol [12, 87, 88], which is one of the potent positive regulators of intestinal calcium absorption [89]. Thus, thalassemic patients experienced thalassemia-induced calciotropic endocrinopathies, leading to decreased levels of calcium transport-regulating hormones including 1,25(OH)2D3, PTH and calcitonin.
Regarding FGF-23, although it has been reported to negate intestinal calcium absorption in mice [31, 32], there are limited studies on the roles of FGF-23 on calcium homeostasis in thalassemia. Most studies focused on the role of FGF-23 on iron metabolism [90,91,92]. For example, Bożentowicz-Wikarek and coworkers reported a low level of circulating iron being associated with an increase in FGF-23 levels [90]. Thus, more understanding about FGF-23 and thalassemia would help in improving bone health in thalassemic patients.
Effects of thalassemia-induced iron hyperabsorption, iron metabolism dysregulation, and iron overload on the intestinal calcium transport
Iron overload in thalassemic patients could have resulted from treatment involving repeated blood transfusion as well as ineffective erythropoiesis and the anemia-induced compensation iron hyperabsorption in the small intestine. In iron-overloading conditions, iron deposit in the solid organs (e.g., liver) and endocrine organs (e.g., pancreas and gonads) could lead to organ damage and endocrine disturbance, respectively [93,94,95,96,97,98,99,100,101,102]. As depicted in Fig. 2, upregulation of iron transporters, and subsequent increased iron absorption were reported in thalassemic patients [103]. Studies in non-transfusion-dependent thalassemic patients [104] and thalassemic mice with moderate anemia [102, 105,106,107] also showed increased intestinal iron absorption. The iron transporters and related proteins that were upregulated in thalassemic mice included divalent metal transporter (DMT)-1 (an apical transporter for iron uptake), ferroportin-1 (a basolateral transporter for iron efflux from the enterocytes), neutral gelatinase-associated lipocalin (NGAL), and transferrin receptor (TfR)-1 [11, 12, 105, 107]. In contrast, the expression of the negative regulator of intestinal iron transport, namely hepcidin, which is normally produced by the liver and binds to ferroportin-1, was decreased in thalassemic mice, and hepcidin treatment could alleviate iron overload in these mice [96, 102, 105,106,107,108]. Thus, the upregulation of iron transporters as well as the downregulation of hepcidin could contribute to an increase in intestinal iron absorption, which would worsen the iron overload condition in thalassemia.
Cellular mechanisms of iron transport in thalassemia. Under normal conditions, dietary iron in the intestinal lumen can traverse the apical membrane by several pathways, such as transferrin receptor 1 (TfR1)-mediated uptake of iron-bound transferrin (Tf), divalent metal transporter 1 (DMT1)-mediated uptake of Fe2+, and Fe-heme uptake by heme carrier protein (HCP)-1. Although Fe3+ (non-heme iron) is more abundant than Fe2+, DMT1 predominantly transports Fe2+ in the presence of H+ in the lumen; therefore, it needs DCytb1 to change the iron redox state. Meanwhile, after endocytosis, iron ions are liberated from Fe-Tf-TfR1 by a metalloreductase, six-transmembrane epithelial antigen of the prostate (STEAP)-3, before being transported into the cytoplasm via DMT1 to join the cellular labile iron pool (LIP). Iron in LIP can be distributed into mitochondria and ferritin, or extruded across the basolateral membrane by ferroportin-1 (FPN). FPN is also a receptor for hepcidin, which can induce FPN internalization and degradation. In thalassemia, the upregulated expressions of DMT1 and TfR1 enhance apical iron uptake into the intestinal epithelial cells. In addition, a reduction in the hepcidin levels decreases FPN internalization and degradation, which can, in turn, increase the number of FPN proteins in the basolateral membrane, and then enhances the intestinal iron absorption
While iron absorption was upregulated in thalassemia [105], many studies showed a significant decrease in calcium absorption as mentioned previously [11, 12]. Recently, an inverse correlation between duodenal calcium transport and iron absorption was demonstrated in thalassemic mice [12]. Subcutaneous administration of hepcidin thus increased calcium transport in these thalassemic mice. In this study, hepcidin showed its potential to effectively alleviate intestinal calcium malabsorption as well as to relieve iron overload by inhibiting intestinal iron transport in thalassemic mice. The mechanism(s) underlying this inverse correlation is not completely understood. Since iron and calcium did not share apical or basolateral transporters, it was likely that the interaction resided in the cytoplasm of intestinal epithelial cells, i.e., the intracellular translocation of their binding proteins or the membrane-bound vesicles [109]. In the duodenums of thalassemic mice, abolishment of hepcidin effects on calcium absorption by a chemical (e.g., chloroquine) that disrupted the function of intracellular vesicles and vesicular transport suggested the possible interaction between calcium and iron transport systems in the vesicles [12]. These intracellular vesicles are believed to rapidly shuttle both calcium and iron from the apical side to basolateral side of the enterocyte. It has been suggested that the lysosome-like intracellular vesicles are able to accommodate iron [110], and they may use Ca2+/H+ exchanger (CAX) to accumulate calcium in exchange with H+ efflux into the cytoplasm [111]. Since the vesicular H+ efflux is dependent on cytoplasmic pH (i.e., acidic pH in the vesicle vs. more alkaline pH in the cytoplasm), an impairment of cellular pH balance may also diminish both calcium and iron absorption. Recently, an inhibitor of Na+/H+ exchanger (NHE)-3, which is essential for cellular pH regulation, was found to hinder the hepcidin-induced calcium transport in the duodenums of BKO mice [58].
Other than thalassemic mice, the reciprocal correlation between calcium absorption and extracellular iron concentration has been shown in human intestinal epithelial Caco-2 cells [112]. Data from this cell line model showed that cellular calcium absorption was increased with the decrease in the extracellular iron concentration, and the opposite trend could be seen when extracellular iron concentration was elevated [112]. Furthermore, the negative effects of iron overload on intestinal calcium transport could also occur through the decreases in calciotropic hormone levels. Levels of vitamin D were apparently lower by ~ 90% in iron-overloaded and multiple transfused thalassemic patients [113]. This suggested another possible consequence of iron overload-induced decrease in intestinal calcium absorption in thalassemic patients through the downregulation of calciotropic hormone, as depicted in Fig. 3. Consequently, bone loss occurs as a result of low blood calcium levels. Moreover, high iron levels can suppress osteoblast activity [114]. Although the reciprocal interaction between intestinal calcium absorption and iron absorption in thalassemia has been elaborated in many studies, more studies are needed to elucidate the connecting mechanisms between the two minerals. Manipulation of the iron transport system by using inhibitors (e.g., DMT1 inhibitor or recombinant hepcidin as a negative regulator of ferroportin-1 function) could be a potential novel intervention to assuage both intestinal calcium malabsorption and iron overload for thalassemic patients.
Reciprocal interaction between calcium and iron absorption in thalassemia induces bone loss. Decreased calcium absorption in thalassemia is caused by a reduction in 1,25(OH)2D3 levels and also by iron hyperabsorption/overload. Low hepcidin levels in thalassemia can basically enhance intestinal iron absorption, which decreases calcium absorption. Since hepcidin has been found to enhance calcium transport in BKO mice, a reduction in its level or action possibly diminishes calcium absorption. Consequently, bone loss occurs as a result of low blood calcium levels. Moreover, high iron levels can suppress osteoblast (OB) activity, thus compromising bone formation
An iron chelator is often prescribed to mitigate iron overload in thalassemic patients. Up till now, there has been no study to investigate the direct effect of iron chelator [e.g., desferoxamine (DFO)] on serum calcium level. The potential association between DFO and hypocalcemia was reported in an infant with parenteral nutrition-associated aluminum overload, which could lead to impaired bone metabolism. The patient failed to respond to any calcium or vitamin D supplement, especially when the level of blood aluminum was high. However, shortly after DFO treatment, the urinary and blood calcium decreased. Accordingly, it was postulated that a decreased serum calcium level could indirectly come from an increased calcium accretion into bone as the level of aluminum was reduced during DFO treatment [115].
On the other hand, some studies have suggested the potential positive effects of iron chelator treatment that capable of improving hypoparathyroidism and hypothyroidism in thalassemia patients [56, 116]. One has shown no correlation between hypothyroidism and the regularity of iron chelation treatment [117]. Others reported no significant side effects on calcium level or calciotropic hormones from oral intake of deferiprone, DFO, or a combination of both in thalassemia patients [118,119,120]. Thus, the effects of iron chelators, particularly DFO, remain controversial and need more investigation.
Conclusions and perspectives
Although thalassemia is a complex genetic disease affecting several organs, including intestine and bone, it is a good model for investigating an association between iron and calcium transport across the intestinal epithelium. Generally, thalassemia with iron hyperabsorption leads to impaired calcium absorption. Negative correlation between iron and calcium transport has recently been demonstrated in the duodenum of thalassemic mice [12], consistent with the general recommendation that iron and calcium supplements should not be administered simultaneously. The thalassemia-induced impairment in calcium transport is caused by several factors, i.e., impaired calciotropic hormone production and response as well as low transcellular calcium uptake. Iron hyperabsorption results, in part, from aberrant hepcidin release and response, and overexpression of DMT1 and/or ferroportin-1. Correlations between serum hepcidin, iron, and other negative regulators of calcium absorption, e.g., FGF-23 [121], remain elusive. Understanding of the underlying mechanism by which iron hinders calcium transport across the intestinal epithelium is crucial for development of better calcium/iron supplement products, particularly for pregnant women who normally need both minerals for fetal development. Finally, hepcidin and iron transport blockers (e.g., DMT1 inhibitor) may be useful for thalassemic patients, who require reduction of iron absorption and restoration of intestinal calcium uptake.
References
Christakos S, Dhawan P, Porta A, Mady LJ, Seth T (2011) Vitamin D and intestinal calcium absorption. Mol Cell Endocrinol 347:25–29
Fleet JC, Eksir F, Hance KW, Wood RJ (2002) Vitamin D-inducible calcium transport and gene expression in three Caco-2 cell lines. Am J Physiol Gastrointest Liver Physiol 283:G618–G625
Jaeger P, Jones W, Clemens TL, Hayslett JP (1986) Evidence that calcitonin stimulates 1,25-dihydroxyvitamin D production and intestinal absorption of calcium in vivo. J Clin Invest 78:456–461
Nemere I, Larsson D (2002) Does PTH have a direct effect on intestine? J Cell Biochem 86:29–34
Wongdee K, Charoenphandhu N (2015) Vitamin D-enhanced duodenal calcium transport. Vitam Horm 98:407–440
Kelly N (2012) Thalassemia. Pediatr Rev 33:434–435
Muncie HL, Campbell J (2009) Alpha and beta thalassemia. Am Fam Physician 80:339–344
Haidar R, Musallam KM, Taher AT (2011) Bone disease and skeletal complications in patients with β thalassemia major. Bone 48:425–432
Nienhuis AW, Nathan DG (2012) Pathophysiology and clinical manifestations of the β-thalassemias. Cold Spring Harb Perspect Med 2:a011726
Liakakos D, Vlachos P, Anoussakis C, Constantinides C, Tsakalosos I (1976) Calcium metabolism in children suffering from homozygous β-thalassaemia after oral administration of 47Ca. Nuklearmedizin 15:77–79
Charoenphandhu N, Kraidith K, Teerapornpuntakit J, Thongchote K, Khuituan P, Svasti S, Krishnamra N (2013) 1,25-Dihydroxyvitamin D3-induced intestinal calcium transport is impaired in β-globin knockout thalassemic mice. Cell Biochem Funct 31:685–691
Kraidith K, Svasti S, Teerapornpuntakit J, Vadolas J, Chaimana R, Lapmanee S, Suntornsaratoon P, Krishnamra N, Fucharoen S, Charoenphandhu N (2016) Hepcidin and 1,25(OH)2D3 effectively restore Ca2+ transport in β-thalassemic mice: reciprocal phenomenon of Fe2+ and Ca2+ absorption. Am J Physiol Endocrinol Metab 311:E214–E223
Carmeliet G, Dermauw V, Bouillon R (2015) Vitamin D signaling in calcium and bone homeostasis: a delicate balance. Best Pract Res Clin Endocrinol Metab 29:621–631
Hagino H (2015) Vitamin D3 analogs for the treatment of osteoporosis. Can J Physiol Pharmacol 93:327–332
Kellett GL (2011) Alternative perspective on intestinal calcium absorption: proposed complementary actions of Cav1.3 and TRPV6. Nutr Rev 69:347–370
Schröder B, Schlumbohm C, Kaune R, Breves G (1996) Role of calbindin-D9k in buffering cytosolic free Ca2+ ions in pig duodenal enterocytes. J Physiol 492:715–722
Timmermans JAH, Bindels RJM, Van Os CH (1995) Stimulation of plasma membrane Ca2+ pump by Calbindin-D28k and calmodulin is additive in EGTA-free solutions. J Nutr 125:1981S–1986S
Walters JR (1989) Calbindin-D9k stimulates the calcium pump in rat enterocyte basolateral membranes. Am J Physiol 256:G124–G128
Charoenphandhu N, Krishnamra N (2007) Prolactin is an important regulator of intestinal calcium transport. Can J Physiol Pharmacol 85:569–581
Charoenphandhu N, Wongdee K, Krishnamra N (2010) Is prolactin the cardinal calciotropic maternal hormone? Trends Endocrinol Metab 21:395–401
Karbach U (1992) Paracellular calcium transport across the small intestine. J Nutr 122:672–677
Tanrattana C, Charoenphandhu N, Limlomwongse L, Krishnamra N (2004) Prolactin directly stimulated the solvent drag-induced calcium transport in the duodenum of female rats. Biochim Biophys Acta 1665:81–91
Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, Yamamoto Y, Wada T, Kojima T, Yokozaki H, Yamashita T, Kato S, Sawada N, Chiba H (2008) Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell 19:1912–1921
Karim MF, Ismail M, Hasan AM, Shekhar HU (2016) Hematological and biochemical status of Beta-thalassemia major patients in Bangladesh: a comparative analysis. Int J Hematol Oncol Stem Cell Res 10:7–12
Kopic S, Geibel JP (2013) Gastric acid, calcium absorption, and their impact on bone health. Physiol Rev 93:189–268
Peacock M (2010) Calcium metabolism in health and disease. Clin J Am Soc Nephrol 5:S23–S30
Picotto G, Massheimer V, Boland R (1997) Parathyroid hormone stimulates calcium influx and the cAMP messenger system in rat enterocytes. Am J Physiol 273:C1349–C1353
Nemere I, Szego CM (1981) Early actions of parathyroid hormone and 1,25-dihydroxycholecalciferol on isolated epithelial cells from rat intestine: I. Limited lysosomal enzyme release and calcium uptake. Endocrinology 108:1450–1462
Chen RA, Goodman WG (2004) Role of the calcium-sensing receptor in parathyroid gland physiology. Am J Physiol Renal Physiol 286:F1005–F1011
Cianferotti L, Gomes AR, Fabbri S, Tanini A, Brandi ML (2015) The calcium-sensing receptor in bone metabolism: from bench to bedside and back. Osteoporos Int 26:2055–2071
Khuituan P, Teerapornpuntakit J, Wongdee K, Suntornsaratoon P, Konthapakdee N, Sangsaksri J, Sripong C, Krishnamra N, Charoenphandhu N (2012) Fibroblast growth factor-23 abolishes 1,25-dihydroxyvitamin D3-enhanced duodenal calcium transport in male mice. Am J Physiol Endocrinol Metab 302:E903–E913
Khuituan P, Wongdee K, Jantarajit W, Suntornsaratoon P, Krishnamra N, Charoenphandhu N (2013) Fibroblast growth factor-23 negates 1,25(OH)2D3-induced intestinal calcium transport by reducing the transcellular and paracellular calcium fluxes. Arch Biochem Biophys 536:46–52
Yoshiko Y, Wang H, Minamizaki T, Ijuin C, Yamamoto R, Suemune S, Kozai K, Tanne K, Aubin JE, Maeda N (2007) Mineralized tissue cells are a principal source of FGF23. Bone 40:1565–1573
Jüppner H (2011) Phosphate and FGF-23. Kidney Int 79121:S24–S27
Kolek OI, Hines ER, Jones MD, LeSueur LK, Lipko MA, Kiela PR, Collins JF, Haussler MR, Ghishan FK (2005) 1α,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol 289:G1036–G1042
Shimada T, Yamazaki Y, Takahashi M, Hasegawa H, Urakawa I, Oshima T, Ono K, Kakitani M, Tomizuka K, Fujita T, Fukumoto S, Yamashita T (2005) Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol 289:F1088–F1095
Skordis N, Toumba M (2011) Bone disease in thalassaemia major: recent advances in pathogenesis and clinical aspects. Pediatr Endocrinol Rev 8:300–306
Wong P, Fuller PJ, Gillespie MT, Milat F (2016) Bone disease in thalassemia: a molecular and clinical overview. Endocr Rev 37:320–346
Soliman AT, El Banna N, Abdel Fattah M, ElZalabani MM, Ansari BM (1998) Bone mineral density in prepubertal children with & #x03B2;-thalassemia: correlation with growth and hormonal data. Metabolism 47:541–548
Benigno V, Bertelloni S, Baroncelli GI, Bertacca L, Di Peri S, Cuccia L, Borsellino Z, Maggio MC (2003) Effects of thalassemia major on bone mineral density in late adolescence. J Pediatr Endocrinol Metab 16:337–342
Mahachoklertwattana P, Chuansumrit A, Sirisriro R, Choubtum L, Sriphrapradang A, Rajatanavin R (2003) Bone mineral density, biochemical and hormonal profiles in suboptimally treated children and adolescents with β-thalassaemia disease. Clin Endocrinol (Oxf) 58:273–279
Merchant R, Udani A, Puri V, D’Cruz V, Patkar D, Karkera A (2010) Evaluation of osteopathy in thalassemia by bone mineral densitometry and biochemical indices. Indian J Pediatr 77:987–991
Soliman A, Adel A, Bedair E, Wagdy M (2008) An adolescent boy with thalassemia major presenting with bone pain, numbness, tetanic contractions and growth and pubertal delay: panhypopituitarism and combined vitamin D and parathyroid defects. Pediatr Endocrinol Rev 6:155–157
Vogiatzi MG, Macklin EA, Fung EB, Vichinsky E, Olivieri N, Kwiatkowski J, Cohen A, Neufeld E, Giardina PJ (2006) Prevalence of fractures among the Thalassemia syndromes in North America. Bone 38:571–575
Vogiatzi MG, Macklin EA, Fung EB, Cheung AM, Vichinsky E, Olivieri N, Kirby M, Kwiatkowski JL, Cunningham M, Holm IA, Lane J, Schneider R, Fleisher M, Grady RW, Peterson CC, Giardina PJ, Thalassemia Clinical Research N (2009) Bone disease in thalassemia: a frequent and still unresolved problem. J Bone Miner Res 24:543–557
Thongchote K, Svasti S, Sa-ardrit M, Krishnamra N, Fucharoen S, Charoenphandhu N (2011) Impaired bone formation and osteopenia in heterozygous βIVSII−654 knockin thalassemic mice. Histochem Cell Biol 136:47–56
Thongchote K, Svasti S, Teerapornpuntakit J, Krishnamra N, Charoenphandhu N (2014) Running exercise alleviates trabecular bone loss and osteopenia in hemizygous β-globin knockout thalassemic mice. Am J Physiol Endocrinol Metab 306:E1406–E1417
Thongchote K, Svasti S, Teerapornpuntakit J, Suntornsaratoon P, Krishnamra N, Charoenphandhu N (2015) Bone microstructural defects and osteopenia in hemizygous βIVSII−654 knockin thalassemic mice: sex-dependent changes in bone density and osteoclast function. Am J Physiol Endocrinol Metab 309:E936–E948
Morabito N, Russo GT, Gaudio A, Lasco A, Catalano A, Morini E, Franchina F, Maisano D, La Rosa M, Plota M, Crifo A, Meo A, Frisina N (2007) The “lively” cytokines network in β-thalassemia major-related osteoporosis. Bone 40:1588–1594
Oztürk O, Yaylim I, Aydin M, Yilmaz H, Agaçhan B, Demiralp E, Isbir T (2001) Increased plasma levels of interleukin-6 and interleukin-8 in β-thalassaemia major. Haematologia (Budap) 31:237–244
Wanachiwanawin W, Wiener E, Siripanyaphinyo U, Chinprasertsuk S, Mawas F, Fucharoen S, Wickramasinghe SN, Pootrakul P, Visudhiphan S (1999) Serum levels of tumor necrosis factor-α, interleukin-1, and interferon-γ in β°-thalassemia/HbE and their clinical significance. J Interferon Cytokine Res 19:105–111
Dundar U, Kupesiz A, Ozdem S, Gilgil E, Tuncer T, Yesilipek A, Gultekin M (2007) Bone metabolism and mineral density in patients with beta-thalassemia major. Saudi Med J 28:1425–1429
Saki N, Abroun S, Salari F, Rahim F, Shahjahani M, Javad MA (2015) Molecular aspects of bone resorption in β-thalassemia major. Cell J 17:193–200
Voskaridou E, Christoulas D, Xirakia C, Varvagiannis K, Boutsikas G, Bilalis A, Kastritis E, Papatheodorou A, Terpos E (2009) Serum Dickkopf-1 is increased and correlates with reduced bone mineral density in patients with thalassemia-induced osteoporosis. Reduction post-zoledronic acid administration. Haematologica 94:725–728
Voskaridou E, Christoulas D, Plata E, Bratengeier C, Anastasilakis AD, Komninaka V, Kaliontzi D, Gkotzamanidou M, Polyzos SA, Dimopoulou M, Terpos E (2012) High circulating sclerostin is present in patients with thalassemia-associated osteoporosis and correlates with bone mineral density. Horm Metab Res 44:909–913
Aleem A, Al-Momen AK, Al-Harakati MS, Hassan A, Al-Fawaz I (2000) Hypocalcemia due to hypoparathyroidism in β-thalassemia major patients. Ann Saudi Med 20:364–366
Goyal M, Abrol P, Lal H (2010) Parathyroid and calcium status in patients with thalassemia. Indian J Clin Biochem 25:385–387
Charoenphandhu N, Kraidith K, Lertsuwan K, Sripong C, Suntornsaratoon P, Svasti S, Krishnamra N, Wongdee K (2017) Na+/H+ exchanger 3 inhibitor diminishes hepcidin-enhanced duodenal calcium transport in hemizygous β-globin knockout thalassemic mice. Mol Cell Biochem 427:201–208
Sharon P, Karmeli F, Rachmilewitz D (1982) Decreased jejunal (Na + K)-ATPase activity in pernicious anemia. Dig Dis Sci 27:1143
Callahan LS, Thibert KA, Wobken JD, Georgieff MK (2013) Early-life iron deficiency anemia alters the development and long-term expression of parvalbumin and perineuronal nets in the rat hippocampus. Dev Neurosci 35:427–436
Matak P, Zumerle S, Mastrogiannaki M, El Balkhi S, Delga S, Mathieu JR, Canonne-Hergaux F, Poupon J, Sharp PA, Vaulont S, Peyssonnaux C (2013) Copper deficiency leads to anemia, duodenal hypoxia, upregulation of HIF-2alpha and altered expression of iron absorption genes in mice. PLoS ONE 8:e59538
Michalitsi V, Dafopoulos K, Gourounti K, Messini C, Ioannou M, Christodoulaki C, Panagopoulos P, Messinis I (2015) Hypoxia-inducible factor-1α (HIF-1α) expression in placentae of women with iron deficiency anemia and β-thalassemia trait. J Matern Fetal Neonatal Med 28:470–474
Ziello JE, Jovin IS, Huang Y (2007) Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med 80:51–60
Wu J, Sun X, Wu Q, Li H, Li L, Feng J, Zhang S, Xu L, Li K, Li X, Wang X, Chen H (2016) Disrupted intestinal structure in a rat model of intermittent hypoxia. Mol Med Rep 13:4407–4413
Zhang J, Wang P, He W, Wang F (2016) Changes in expression of Slingshot protein in hypoxic human intestinal epithelial cell and its relation with barrier function of the cells. Zhonghua Shao Shang Za Zhi 32:249–253
Okano T, Tsugawa N, Morishita A, Kato S (2004) Regulation of gene expression of epithelial calcium channels in intestine and kidney of mice by 1α,25-dihydroxyvitamin D3. J Steroid Biochem Mol Biol 89–90:335–338
Ghijsen WE, De Jong MD, Van Os CH (1983) Kinetic properties of Na+/Ca2+ exchange in basolateral plasma membranes of rat small intestine. Biochim Biophys Acta 730:85–94
Centeno V, Picotto G, Perez A, Alisio A, Tolosa de Talamoni N (2011) Intestinal Na+/Ca2+ exchanger protein and gene expression are regulated by 1,25(OH)2D3 in vitamin D-deficient chicks. Arch Biochem Biophys 509:191–196
Aloia JF, Ostuni JA, Yeh JK, Zaino EC (1982) Combined vitamin D parathyroid defect in thalassemia major. Arch Intern Med 142:831–832
de Vernejoul MC, Girot R, Gueris J, Cancela L, Bang S, Bielakoff J, Mautalen C, Goldberg D, Miravet L (1982) Calcium phosphate metabolism and bone disease in patients with homozygous thalassemia. J Clin Endocrinol Metab 54:276–281
Napoli N, Carmina E, Bucchieri S, Sferrazza C, Rini GB, Di Fede G (2006) Low serum levels of 25-hydroxyvitamin D in adults affected by thalassemia major or intermedia. Bone 38:888–892
Zamboni G, Marradi P, Tagliaro F, Dorizzi R, Tato L (1986) Parathyroid hormone, calcitonin and vitamin D metabolites in beta-thalassaemia major. Eur J Pediatr 145:133–136
Chapelon E, Garabedian M, Brousse V, Souberbielle JC, Bresson JL, de Montalembert M (2009) Osteopenia and vitamin D deficiency in children with sickle cell disease. Eur J Haematol 83:572–578
Pratelli L, Verri E, Fortini M, Marconi S, Zolezzi C, Fornasari PM, Gamberini MR, De Sanctis V (2006) Chelation therapy and bone metabolism markers in thalassemia major. J Pediatr Endocrinol Metab 19:1335–1342
Soliman A, Adel A, Wagdy M, Al Ali M, ElMulla N (2008) Calcium homeostasis in 40 adolescents with β-thalassemia major: a case-control study of the effects of intramuscular injection of a megadose of cholecalciferol. Pediatr Endocrinol Rev 6:149–154
Costin G, Kogut MD, Hyman CB, Ortega JA (1979) Endocrine abnormalities in thalassemia major. Am J Dis Child 133:497–502
El-Nashar M, Mortagy AK, El-Beblawy NMS, El-Gohary E, Kamel IM, Rashad M, Mouharam WA (2017) Parathyroid hormone in pediatric patients with β-thalassemia major and its relation to bone mineral density; a case control study. Egypt J Med Hum Genet 18:75–78
Jensen CE, Tuck SM, Old J, Morris RW, Yardumian A, De Sanctis V, Hoffbrand AV, Wonke B (1997) Incidence of endocrine complications and clinical disease severity related to genotype analysis and iron overload in patients with & #x03B2;-thalassaemia. Eur J Haematol 59:76–81
Bouillon R, Van Cromphaut S, Carmeliet G (2003) Intestinal calcium absorption: molecular vitamin D mediated mechanisms. J Cell Biochem 88:332–339
Van Cromphaut SJ, Rummens K, Stockmans I, Van Herck E, Dijcks FA, Ederveen AG, Carmeliet P, Verhaeghe J, Bouillon R, Carmeliet G (2003) Intestinal calcium transporter genes are upregulated by estrogens and the reproductive cycle through vitamin D receptor-independent mechanisms. J Bone Miner Res 18:1725–1736
Nemere I, Norman AW (1989) 1,25-Dihydroxyvitamin D3-mediated vesicular calcium transport in intestine: dose-response studies. Mol Cell Endocrinol 67:47–53
Olson EB Jr, Deluca HF, Potts JT Jr (1972) Calcitonin inhibition of vitamin D-induced intestinal calcium absorption. Endocrinology 90:151–157
Cramer CF (1973) Effect of salmon calcitonin on in vivo calcium absorption in rats. Calcif Tissue Res 13:169–172
Swaminathan R, Ker J, Care D (1974) Calcitonin and intestinal calcium absorption. J Endocrinol 61:83–94
Wongsurawat N, Armbrecht HJ (1991) Calcitonin stimulates 1,25-dihydroxyvitamin D production in diabetic rat kidney. Metabolism 40:22–25
Canatan D, Akar N, Arcasoy A (1995) Effects of calcitonin therapy on osteoporosis in patients with thalassemia. Acta Haematol 93:20–24
Lasco A, Morabito N, Gaudio A, Buemi M, Wasniewska M, Frisina N (2001) Effects of hormonal replacement therapy on bone metabolism in young adults with beta-thalassemia major. Osteoporos Int 12:570–575
Tiosano D, Hochberg Z (2001) Endocrine complications of thalassemia. J Endocrinol Invest 24:716–723
Colin EM, Van Den Bemd GJ, Van Aken M, Christakos S, De Jonge HR, Deluca HF, Prahl JM, Birkenhager JC, Buurman CJ, Pols HA, Van Leeuwen JP (1999) Evidence for involvement of 17β-estradiol in intestinal calcium absorption independent of 1,25-dihydroxyvitamin D3 level in the rat. J Bone Miner Res 14:57–64
Bożentowicz-Wikarek M, Kocełak P, Owczarek A, Olszanecka-Glinianowicz M, Mossakowska M, Skalska A, Więcek A, Chudek J (2015) Plasma fibroblast growth factor 23 concentration and iron status. Does the relationship exist in the elderly population? Clin Biochem 48:431–436
Hanudel MR, Chua K, Rappaport M, Gabayan V, Valore E, Goltzman D, Ganz T, Nemeth E, Salusky IB (2016) Effects of dietary iron intake and chronic kidney disease on fibroblast growth factor 23 metabolism in wild-type and hepcidin knockout mice. Am J Physiol Renal Physiol 311:F1369–F1377
Wolf M, Koch TA, Bregman DB (2013) Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res 28:1793–1803
Abdulzahra MS, Al-Hakeim HK, Ridha MM (2011) Study of the effect of iron overload on the function of endocrine glands in male thalassemia patients. Asian J Transfus Sci 5:127–131
de Montalembert M, Ribeil JA, Brousse V, Guerci-Bresler A, Stamatoullas A, Vannier JP, Dumesnil C, Lahary A, Touati M, Bouabdallah K, Cavazzana M, Chauzit E, Baptiste A, Lefebvre T, Puy H, Elie C, Karim Z, Ernst O, Rose C (2017) Cardiac iron overload in chronically transfused patients with thalassemia, sickle cell anemia, or myelodysplastic syndrome. PLoS One 12:e0172147
De Sanctis V, Soliman AT, Elsedfy H, Albu A, Al Jaouni S, Anastasi S, Bisconte MG, Canatan D, Christou S, Daar S, Di Maio S, El Kholy M, Khater D, Elshinawy M, Kilinc Y, Mattei R, Mosli HH, Quota A, Roberti MG, Sobti P, Yaarubi SA, Canpisi S, Kattamis C (2017) Review and recommendations on management of adult female thalassemia patients with hypogonadism based on literature review and experience of ICET-A network specialists. Mediterr J Hematol Infect Dis 9:e2017001
Gardenghi S, Ramos P, Follenzi A, Rao N, Rachmilewitz EA, Giardina PJ, Grady RW, Rivella S (2010) Hepcidin and Hfe in iron overload in β-thalassemia. Ann N Y Acad Sci 1202:221–225
Jackson LH, Vlachodimitropoulou E, Shangaris P, Roberts TA, Ryan TM, Campbell-Washburn AE, David AL, Porter JB, Lythgoe MF, Stuckey DJ (2017) Non-invasive MRI biomarkers for the early assessment of iron overload in a humanized mouse model of β-thalassemia. Sci Rep. https://doi.org/10.1038/srep43439
Karami H, Kosaryan M, Amree AH, Darvishi-Khezri H, Mousavi M (2017) Combination iron chelation therapy with deferiprone and deferasirox in iron-overloaded patients with transfusion-dependent β-thalassemia major. Clin Pract 7:912
Limenta LM, Jirasomprasert T, Jittangprasert P, Wilairat P, Yamanont P, Chantharaksri U, Fucharoen S, Morales NP (2011) Pharmacokinetics of deferiprone in patients with & #x03B2;-thalassaemia: impact of splenectomy and iron status. Clin Pharmacokinet 50:41–50
Mishra AK, Tiwari A (2013) Iron overload in beta thalassaemia major and intermedia patients. Maedica (Buchar) 8:328–332
Musallam KM, Cappellini MD, Taher AT (2013) Iron overload in β-thalassemia intermedia: an emerging concern. Curr Opin Hematol 20:187–192
Ramos P, Melchiori L, Gardenghi S, Van-Roijen N, Grady RW, Ginzburg Y, Rivella S (2010) Iron metabolism and ineffective erythropoiesis in & #x03B2;-thalassemia mouse models. Ann N Y Acad Sci 1202:24–30
Fiorelli G, Fargion S, Piperno A, Battafarano N, Cappellini MD (1990) Iron metabolism in thalassemia intermedia. Haematologica 75:89–95
Taher AT, Porter J, Viprakasit V, Kattamis A, Chuncharunee S, Sutcharitchan P, Siritanaratkul N, Galanello R, Karakas Z, Lawniczek T, Ros J, Zhang Y, Habr D, Cappellini MD (2012) Deferasirox reduces iron overload significantly in nontransfusion-dependent thalassemia: 1-year results from a prospective, randomized, double-blind, placebo-controlled study. Blood 120:970–977
Gardenghi S, Marongiu MF, Ramos P, Guy E, Breda L, Chadburn A, Liu Y, Amariglio N, Rechavi G, Rachmilewitz EA, Breuer W, Cabantchik ZI, Wrighting DM, Andrews NC, de Sousa M, Giardina PJ, Grady RW, Rivella S (2007) Ineffective erythropoiesis in β-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood 109:5027–5035
Gardenghi S, Ramos P, Marongiu MF, Melchiori L, Breda L, Guy E, Muirhead K, Rao N, Roy CN, Andrews NC, Nemeth E, Follenzi A, An X, Mohandas N, Ginzburg Y, Rachmilewitz EA, Giardina PJ, Grady RW, Rivella S (2010) Hepcidin as a therapeutic tool to limit iron overload and improve anemia in β-thalassemic mice. J Clin Invest 120:4466–4477
Weizer-Stern O, Adamsky K, Amariglio N, Rachmilewitz E, Breda L, Rivella S, Rechavi G (2006) mRNA expression of iron regulatory genes in β-thalassemia intermedia and β-thalassemia major mouse models. Am J Hematol 81:479–483
Bartnikas TB, Fleming MD (2010) A tincture of hepcidin cures all: the potential for hepcidin therapeutics. J Clin Invest 120:4187–4190
Wasserman RH, Chandler JS, Meyer SA, Smith CA, Brindak ME, Fullmer CS, Penniston JT, Kumar R (1992) Intestinal calcium transport and calcium extrusion processes at the basolateral membrane. J Nutr 122:662–671
Moriya M, Linder MC (2006) Vesicular transport and apotransferrin in intestinal iron absorption, as shown in the Caco-2 cell model. Am J Physiol Gastrointest Liver Physiol 290:G301–G309
Lloyd-Evans E (2016) On the move, lysosomal CAX drives Ca2+ transport and motility. J Cell Biol 212:755–757
Wang L, Li Q, Duan XL, Chang YZ (2005) Effects of extracellular iron concentration on calcium absorption and relationship between Ca2+ and cell apoptosis in Caco-2 cells. World J Gastroenterol 11:2916–2921
Merchant RH, Shirodkar A, Ahmed J (2011) Evaluation of growth, puberty and endocrine dysfunctions in relation to iron overload in multi transfused Indian thalassemia patients. Indian J Pediatr 78:679–683
He YF, Ma Y, Gao C, Zhao GY, Zhang LL, Li GF, Pan YZ, Li K, Xu YJ (2013) Iron overload inhibits osteoblast biological activity through oxidative stress. Biol Trace Elem Res 152:292–296
Klein GL, Snodgrass WR, Griffin MP, Miller NL, Alfrey AC (1989) Hypocalcemia complicating deferoxamine therapy in an infant with parenteral nutrition-associated aluminum overload: evidence for a role of aluminum in the bone disease of infants. J Pediatr Gastroenterol Nutr 9:400–403
Chirico V, Lacquaniti A, Salpietro V, Luca N, Ferrau V, Piraino B, Rigoli L, Salpietro C, Arrigo T (2013) Thyroid dysfunction in thalassaemic patients: ferritin as a prognostic marker and combined iron chelators as an ideal therapy. Eur J Endocrinol 169:785–793
Eshragi P, Tamaddoni A, Zarifi K, Mohammadhasani A, Aminzadeh M (2011) Thyroid function in major thalassemia patients: is it related to height and chelation therapy? Casp J Intern Med 2:189–193
Rombos Y, Tzanetea R, Konstantopoulos K, Simitzis S, Zervas C, Kyriaki P, Kavouklis M, Aessopos A, Sakellaropoulos N, Karagiorga M, Kalotychou V, Loukopoulos D (2000) Chelation therapy in patients with thalassemia using the orally active iron chelator deferiprone (L1). Haematologica 85(2):115–117
Fisher SA, Brunskill SJ, Doree C, Chowdhury O, Gooding S, Roberts DJ (2013) Oral deferiprone for iron chelation in people with thalassaemia. Cochrane Database Syst Rev CD004839
Fisher SA, Brunskill SJ, Doree C, Gooding S, Chowdhury O, Roberts DJ (2013) Desferrioxamine mesylate for managing transfusional iron overload in people with transfusion-dependent thalassaemia. Cochrane Database Syst Rev CD004450
Rodrat M, Wongdee K, Panupinthu N, Thongbunchoo J, Teerapornpuntakit J, Krishnamra N, Charoenphandhu N (2018) Prolonged exposure to 1,25(OH)2D3 and high ionized calcium induces FGF-23 production in intestinal epithelium-like Caco-2 monolayer: a local negative feedback for preventing excessive calcium transport. Arch Biochem Biophys 640:10–16
Acknowledgements
The authors would like to thank Prof. Nateetip Krishnamra for critical comments and proofreading of the manuscript.
Funding
N. Charoenphandhu has been supported by the Thailand Research Fund (TRF) through TRF Senior Research Scholar Grant (RTA6080007). K. Wongdee has been supported by TRF International Research Network (IRN) Grant (IRN60W0001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
About this article
Cite this article
Lertsuwan, K., Wongdee, K., Teerapornpuntakit, J. et al. Intestinal calcium transport and its regulation in thalassemia: interaction between calcium and iron metabolism. J Physiol Sci 68, 221–232 (2018). https://doi.org/10.1007/s12576-018-0600-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12576-018-0600-1