Abstract
We investigated the cellular mechanisms and therapeutic effect of post-injury stretch on the recovery process from muscle injury induced by lengthening contractions (LC). One day after LC, a single 15-min bout of muscle stretch was applied at an intensity of 3 mNm. The maximal isometric torque was measured before and at 2–21 days after LC. The myofiber size was analyzed at 21 days after LC. Developmental myosin heavy chain-immunoreactive (dMHC-ir) cells, a marker of regenerating myofibers, were observed in the early recovery stage (2–5 days after LC). We observed that LC-induced injury markedly decreased isometric torque and myofiber size, which recovered faster in rats that underwent stretch than in rats that did not. Regenerating myofiber with dMHC-ir cells was observed earlier in rats that underwent stretch. These results indicate that post-injury stretch may facilitate the regeneration and early formation of new myofibers, thereby promoting structural and functional recovery from LC-induced muscle injury.
Similar content being viewed by others
Introduction
Unaccustomed strenuous exercise can result in muscle injury such as structural damage and functional deficit [1]. Compared to other types of muscle contraction, such as shortening or isometric contraction, lengthening contractions (LC), whereby the contracting muscle is being forcibly stretched, are known to cause more severe muscle injury [1,2,3]. Muscle damage restricts activities of daily living and deteriorates the performance of athletes; consequently, the effective prevention and treatment of muscle damage after LC are necessary.
In our recent study, Mori et al. developed a rat model of muscle damage induced by LC [4]. Using this model, we demonstrated that myofiber damage (in strict terms, increased membrane permeability of myofibers, as revealed by labeling with Evans blue dye infiltrated in a fiber) and isometric force decline, occurred with increasing angular velocity of muscle stretch during LC [4]. The structural damage was highly correlated with the decrease in the isometric force production from the muscles that underwent LC. Peak torque generated during LC also increased with the angular velocity of muscle stretch [4]. Using the same device for loading LC, Hayashi and colleagues reported that the magnitude of delayed-onset mechanical hyperalgesia of the muscle increased with the angular velocity and stretch range of the LC [5]. The rate of torque increase and integrated torque quantity generated during LC were the mechanical factors responsible for the angular velocity- and stretch range-dependent mechanical hyperalgesia, respectively [5]. These results indicate that triggering factors in the initiation of LC-induced muscle injury and delayed-onset muscular mechanical hyperalgesia are mechanical in nature [4,5,6,7].
However, passive stretch applied to the mouse extensor digitorum longus muscle before LC provides some protection against LC-induced muscle injury [8]. Passive stretch applied to the rat soleus muscle prior to weight bearing after immobilization of the paw protects the muscle from injury [9]. Together these results suggest that muscle injury could be prevented by preemptive stretch applied to the muscle.
The therapeutic effect of running or stretching on recovery after muscle injury has been reported. In one study, mechanical loading onto myotoxic notexin-injected muscles, resulting from voluntary or forced running, promoted the recovery process in rats [10]. In another study using mice, ultrasound applied to lacerated muscle was found to enhance myofiber regeneration, with increased physiological performance [11]. There is as yet no study demonstrating the therapeutic effect of stretch on LC-induced muscle injury. In addition, the cellular mechanisms of the therapeutic effect of stretch are largely unknown.
In the study reported here, we examined the effects of stretch on the muscle recovery process using a rat model of LC-induced muscle injury, as established in our recent study [4]. Special attention was paid to the histological appearance of the newly formed myofibers labeled with developmental myosin heavy chain (dMHC) immunoreactivity. Muscle function was tested by measuring maximal isometric torque to examine the therapeutic effect of muscle stretch.
Methods
Animals
A total of 114 male Wistar rats (8 weeks old; 225–265 g; SLC Inc., Shizuoka, Japan) were used in the study. The rats were housed two per cage in a clean air-conditioned room (ambient temperature 23 °C) under a 12/12-h light/dark cycle (lights on 0800 to 2000 hour), with free access to food and water throughout the experiment. At the end of the muscle function test in vivo, the rats were sacrificed by cervical dislocation, and samples were taken for immunohistochemical analysis. The study was conducted according to the Regulations for Animal Experiments in Nagoya University and the Fundamental Guidelines for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions in Japan.
Lengthening contractions
With the rats under inhalation anesthesia with 1.5% isoflurane, the ankle extensor muscles were subjected to repetitive LC by using a customized device (NDH-1; Bio Research Center, Nagoya, Japan), as described in our previous study [4]. Briefly, the left extensor muscles were transcutaneously stimulated using a pair of surface electrodes (diameter 3 mm; NM-319Y, Nihon Kohden Corp., Tokyo, Japan) fixed with quick-drying glue to the shaved skin covering the mid-belly of the tibialis anterior (TA) muscle. Maximal tetanic contractions of the ankle extensors were induced by a supramaximal constant current (5 mA; 1-ms pulses at 100 Hz) applied via an isolator (SS-202J; Nihon Kohden Corp., Tokyo, Japan) connected to an electrical stimulator (SEN-3301; Nihon Kohden Corp., Tokyo, Japan). LC were repeatedly induced at an angular velocity of 200°/s, with simultaneous stimulus trains of 650 ms. There was a 200-ms delay from the start of electrical stimulation to the onset of active torque generation of LC. The stretch range of LC was set at 90°, starting with the ankle joint dorsiflexed at 30° and finishing with the ankle joint plantarflexed at 60°. Ten LCs were loaded every 10 s in one set. A total of five sets were conducted per rat at 60-s intervals [4].
Muscle stretch
The rats were placed on their sides on the table under inhalation anesthesia with 1.5% isoflurane (Fig. 1A). The extensor muscles, including the TA, were subjected to repetitive stretch stimulation using the same customized device as that used for loading LC (NDH-1; Bio Research Center, Co., Ltd.). The device enabled us to control stretch variables such as velocity, range, timing, and force. Three axes were set so that the range of motion during stretch stimulation could be precisely defined (Fig. 1B): the femoral axis (between the third trochanter and lateral condyle of the femur); the fibular axis (between the head and lateral malleolus of the fibula); the foot axis (along the base of the metatarsal). The knee and ankle joints were set at 90° before commencing the stretch along the above-defined axes. The center of the moment arm of the ankle joint during muscle stretch was set at 3 mm in front of the lateral malleolus of the fibula.
Experimental device and protocol for passive muscle stretch in a rat model of muscle injury induced by lengthening contractions (LC). A A customized experimental device for passive muscle stretch and isometric torque measurement, consisting of: a a footplate attached to the paw, b a rotary stepping motor, c a control box for driving signals from a computer, d a torque sensor. B Joint-angle settings for passive muscle stretch (i.e., starting position). C Original recording of passive torque elicited during muscle stretch (3 mNm for 15 min). D Enlarged image of the torque curve corresponding to 5 muscle stretches (asterisk in C). Muscle stretch was performed at a stretch velocity of 50°/s for 5 s, followed by a 5-s rest period. E Torque generated during passive stretch at the velocity of 50°/s and various stretch angles. n = 3 rats
In our preliminary experiments, we tested various stretch protocols with different timings or frequencies (e.g., a single bout of stretch immediately or 1 day after LC, or repeated bouts of stretch every day or every 2 days from 3–20 days after LC). However, the extent of structural and functional recovery from LC-induced muscle injury did not differ among the protocols tested. Thus, we chose only one stretch protocol for further analysis, namely the protocol based on a single bout of stretch on the first day after LC.
One day after LC, ramp and hold stretch stimuli were repetitively and automatically applied using the customized device in the form of plantarflexion of the ankle joint at a velocity of 50°/s for 15 min (Fig. 1C). Stretch stimulation was applied at an intensity of 3 mNm for 5 s, followed by a 5-s rest period (Fig. 1D). Stretch intensity was determined according to passive torque generated during muscle stretch in the range of ankle joint plantarflexion (Fig. 1E). A steeper increase in the torque above 3 mNm in the stretch range was observed and assumed to be related to the stretch reflex or the stiffness of tissues other than muscles, such as tendons, ligaments, and joints.
The rats were assigned to three groups: (1) Control group, exposed neither to LC nor to muscle stretch; (2) LC-alone group, exposed to LC, but not to muscle stretch; (3) LC+Stretch group, exposed to both LC and muscle stretch.
Muscle function test
To investigate whether muscle stretch would promote the recovery of muscle function, the maximal isometric torque induced by the dorsiflexion of the ankle joint was measured with the rats under inhalation anesthesia with 1.5% isoflurane. Isometric contractions without muscle stretch were induced using the same device (NDH-1; Bio Research Center, Co., Ltd.) and the same electrical stimulation protocol as those used for loading LC (i.e., 650-ms train duration; 1-ms pulses at 100 Hz; a constant current of 5 mA). The measurement was performed at time 0 (immediately before LC) and at 2, 7, 14, 18, and 21 days after LC. Since the torque generated by muscle contraction increases with body weight, the absolute torque value measured by a sensor equipped with the customized device (Fig. 1A) was corrected by the body weight of the rat and then normalized by the corrected value obtained immediately before LC (day 0).
Hematoxylin and eosin staining
Two days after LC, when muscle damage was obvious, rats were anesthetized with 1.5% isoflurane and the TA was quickly excised from the surrounding tissues without fixation. The muscle was rinsed in physiological saline and blotted dry by using a filter paper. A middle portion of the TA was snap frozen and stored at −80 °C for subsequent histological and immunohistochemical experiments. Transverse sections with a thickness of 10 μm were cut, mounted on slides, and stained with hematoxylin and eosin (H&E) for histological observation. Nine rats were used for this analysis.
Immunohistochemistry
Twenty-one days after LC, the rats were anesthetized with 1.5% isoflurane and the TA was quickly excised from the surrounding tissues. The muscle was rinsed in physiological saline, blotted dry by using filter paper, weighed, and snap frozen. Transverse sections from the middle portion of the TA were cut at a thickness of 5 µm. Sections were washed in phosphate buffered saline (PBS) for 10 min, permeabilized in 0.5% Triton/PBS for 10 min, blocked with 1% bovine serum albumin (BSA)/PBS (1 mg/mL) for 1 h, and incubated in rabbit anti-dystrophin polyclonal primary antibody (1:400; SC-15376; Santa Cruz Biotechnology, Dallas, TX) for 1 h at room temperature. After a subsequent washing with 1% BSA/PBS, immunolabeled sections were incubated for 1 h with a goat anti-rabbit immunoglobulin G (IgG) antibody conjugated to Alexa Fluor® 568 (1:400; A11036; Molecular Probes, Eugene, OR). The sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; 1:10000; D-9564; Sigma-Aldrich, St. Louis, MO) to visualize muscle nuclei. After rinsing, the sections were mounted in 90% glycerol/PBS. In order to quantitatively analyze the stretch-induced histological changes in myofibers, images of the cross-sectional area (CSA) of the muscle belly were acquired using fluorescence microscopy (Nano Zoomer RS 2.0; Hamamatsu Photonics, Shizuoka, Japan). The size (CSA) and number of myofibers with membranes immunostained for dystrophin, a protein specifically located at the cell membrane, were counted and measured using ImageJ software (free software developed by the National Institutes of Health, Bethesda, MD). The analysis was performed on the CSA taken from the superficial, middle, and deep layers of the TA (500 × 500 µm = 250,000 µm2 each). The mean value of the three layers in each section was calculated and used as the CSA of the rat.
Since the maximal isometric contraction torque (i.e., muscle function) recovered faster after muscle stretch, we expected that stretch stimulation could promote recovery from muscle damage. To elucidate the mechanism, we examined histological changes in the early stage of recovery after muscle damage. The muscle specimens were prepared as mentioned above from samples collected at 1, 2, 3, and 4 days after muscle stretch (i.e., at 2, 3, 4, and 5 days after LC, respectively). The cut sections were immunostained with mouse monoclonal anti-dMHC antibody (1:100; Vector Laboratories, Burlingame, CA) and visualized using Alexa Fluor® 488 goat anti-mouse IgG (1:400; A11029; Molecular Probes). The cell membrane and nuclei of myofibers were labeled with anti-dystrophin antibody and DAPI, as described above. The number and size (CSA) of dystrophin-labeled myofibers, regardless of the immunoreactivity to dMHC, were counted and measured using ImageJ software.
Statistical analyses
Results are expressed as mean ± standard error of the mean. The maximal isometric torque was analyzed using two-way repeated measures analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. Immunohistochemical data were compared among groups using one-way ANOVA followed by Tukey’s post hoc test. The proportion of dMHC-ir myofibers in the intervention groups (LC-alone and LC+Stretch) was analyzed using Fisher’s exact probability test. p < 0.05 was considered to indicate statistical significance.
Results
Rat body weight
Because maximal isometric torque is affected by body weight fluctuations, we matched the three groups of rats by body weight at the beginning of the experiment. Body weight was monitored continuously during the 21 days of the experiment, with measurements taken before LC and at 2, 7, 14, 18, and 21 days after LC (Table 1a). There was no significant difference in body weight among three groups (p > 0.05; two-way repeated measures ANOVA).
LC-induced muscle damage
Using H&E staining, we examined if there were any signs of histological damage after applying the LC according to the prescribed protocol (velocity 200°/s; range 90°; repetition 50 times; n = 9 rats). Figure 2 is a typical section taken from the mid-belly of the entire TA at 2 days after repetitive LC, showing necrotic myofibers and infiltration of accumulating mononuclear cells in the entire TA, with slightly higher infiltration in the superficial layer than in the deeper layer of the TA.
Lengthening contraction-induced structural damage in the muscle. a Hematoxylin–eosin staining of the entire cross-section cut from the mid-belly of the tibialis anterior muscle (TA) at 2 days after LC. S, D, M, and L; Superficial, deep, medial, and lateral side of the TA, respectively. b Enlarged image of the area (box in a indicated by the arrow. Necrotic myofibers and infiltration of accumulating mononuclear cells are noted
Muscle function test
The maximal isometric torque induced by electrical stimulation of the ankle extensors was measured before LC and at 2, 7, 14, 18, and 21 days after LC to examine whether stretch stimulation could promote functional recovery from injury. The absolute values of the maximal isometric torque are listed in Table 1b. Compared with the Control group, the normalized isometric torque at 2 days after LC had decreased significantly from the baseline values (down to 23–24% of baseline values) in both the LC-alone and LC+Stretch groups (p < 0.01, two-way ANOVA followed by Tukey’s post hoc test; Fig. 3). The torque gradually returned to the level of the Control group, but the recovery appeared to be faster in the LC+Stretch group than in the LC-alone group. There were significant differences in isometric torque between the LC-alone and LC+Stretch groups on the 7th, 18th, and 21st day after LC (p < 0.05, two-way ANOVA followed by Tukey’s post hoc test). On the 21st day after LC, no significant difference was detected between the LC+Stretch group and the Control group (Fig. 3).
Functional recovery aided by passive muscle stretch after injury induced by lengthening contractions (LC). Maximal isometric torque measured by supramaximal transcutaneous electrical stimulation of the ankle extensor muscles. Muscle stretch was performed in a single 15-min bout at 1 day after LC (indicated by an arrow on the x-axis). Rats exposed to LC were divided into two groups according to whether they underwent stretch (LC-alone vs. LC+Stretch). A control group consisting of rats not exposed to LC was also included (Control). Compared to the values in the Control group, torque was markedly decreased in the LC-alone and LC+Stretch groups at 2 days after LC, but the LC+Stretch group showed faster recovery. Significance at *p < 0.05 and **p < 0.01 vs. LC-alone; †† p < 0.01 vs. Control. p values were computed by two-way repeated measures analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. On day 14, no significant difference in torque was adventitiously detected between the LC-alone and LC+Stretch groups
Size of myofibers
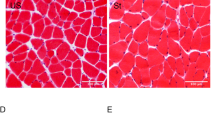
In our recent study, we showed that the CSA of type IIb myofibers was more than twice as large as that of other types of myofibers (i.e., types I and IIa) and that LC-induced muscle damage occurred preferentially in type IIb myofibers [4]. Thus, we expected that the size of myofibers in the TA would decrease after LC and that myofiber size is a useful index for assessing histological recovery. As we noted that the maximal isometric torque returned to the control levels by day 21 in the LC+Stretch group, we assessed the size of myofibers in the TA samples removed at 21 days after LC. In the Control group, myofibers had a normal polygonal shape with different sizes (Fig. 4a). Myofibers in the LC-alone group were oval-like in shape, with smaller sizes (Fig. 4b). In the LC+Stretch group, the size and shape appeared to return to normal at 21 days after LC (Fig. 4c).
Structural recovery aided by passive stretch after muscle injury induced by LC. Muscle stretch was performed in a single 15-min bout at 1 day after LC. Rats exposed to LC were divided into two groups as described in the caption to Fig. 3 (LC-alone vs. LC+Stretch). A control group (described in Fig. 3 caption) was also included (Control). a–c Photomicrographs of the mid-belly of the TA in the Control (a), LC-alone (b), and LC+Stretch (c) groups. The membrane of the myofibers is labeled with anti-dystrophin antibody. d Cross-sectional area (CSA) of the TA at 21 days after LC appears to be significantly smaller in the LC-alone group than in the Control and LC+Stretch groups, for which cross-sectional areas are comparable. Significance at *p < 0.05 and **p < 0.01 by one-way ANOVA followed by Tukey’s multiple comparison test; n.s. not significant
The CSA of myofibers was 3158.3 ± 64.4 μm2 in the Control group (n = 13, Fig. 4d). It was significantly smaller in the LC-alone group (2629.4 ± 137.6 μm2; n = 11) than in the Control group (p < 0.01, one-way ANOVA followed by Tukey’s post hoc test). In the LC+Stretch group, the decreased CSA noted after LC had recovered significantly by the 21st day after LC (3033.1 ± 103.5 μm2; n = 12; p < 0.05, one-way ANOVA followed by Tukey’s post hoc test, Fig. 4d).
Formation of dMHC-ir myofibers
Since functional recovery was observed by the seventh day after LC (i.e., 6th day after muscle stretch), we examined the early appearance of myofibers labeled with dMHC-ir, a marker of myofiber regeneration, at 2–5 days after LC (i.e., 1–4 days after muscle stretch). In the LC-alone and LC+Stretch groups, muscle damage was observed at 2 days after LC in the form of necrotic myofibers and the infiltration of mononuclear cells; muscle damage was still evident at 5 days after LC (Fig. 5A). In the LC-alone group, dMHC-ir myofibers with small diameters were apparent on the fifth day after LC. However, in the LC+Stretch group, dMHC-ir myofibers were clearly observed even on the third and fourth day after LC. Size distribution analysis revealed that the small-sized dMHC-ir myofibers (<800 μm2) appeared earlier in the LC+Stretch group than in the LC-alone group (Fig. 5B). On the third and fourth day after LC, the proportion of newly formed myofibers with dMHC-ir was significantly higher in the LC+Stretch group than in the LC-alone group (p < 0.001, Fisher’s exact probability test; Fig. 5C).
Regeneration aided by passive stretch after muscle damage induced by LC. Muscle stretch was performed in a single 15-min bout at 1 day after LC. Rats exposed to LC were divided into two groups according to whether they underwent stretch (LC-alone vs. LC+Stretch). A Photomicrographs of the TA muscle (a–d LC-alone; e–h LC+Stretch) obtained at various times after LC-induced injury (a, e 2 days, b, f 3 days, c, g 4 days, d, h 5 days). Green Myosin heavy chain-immunoreactive myofibers (dMHC-ir), red dystrophin-ir myofiber membrane, blue nuclei labeled with 4′,6-diamidino-2-phenylindole. Scale bar 50 μm. B Proportion of myofibers containing dystrophin-ir and dMHC-ir, with labeling (a–h) corresponding to those in A. Bin width: CSA of 400 μm2. C Proportion of myofibers with dMHC-ir. On the third and fourth days after LC-induced injury, there was significantly more dMHC-ir myofibers, indicating myofiber regeneration, in the LC+Stretch group than in the LC-alone group (***p < 0.001, Fisher’s exact probability test)
Discussion
In the study described here, we demonstrated that a single bout of repetitive stretch at an intensity of 3 mNm for 15 min on the first day after LC facilitated recovery from LC-induced muscle injury. The maximal isometric torque and size of myofibers, which decreased following LC, recovered to control levels by day 21 in rats that underwent stretch, but not in rats that did not. The faster recovery from injury noted in rats that underwent stretch may have been facilitated by post-injury stretch, which aided the muscle regeneration process, as revealed by the earlier formation of dMHC-ir myofibers.
In their mouse model, McCully and Faulkner have reported that force decline induced by LC recovered within 30 days [2]. In the present study, we observed that the normalized torque decreased after LC and recovered to 80% by day 21 in rats that did not undergo muscle stretch, compared to recovery to 91% in rats that underwent a single bout of muscle stretch. Already by day 7, the torque was significantly higher in rats that underwent stretch than in those that did not. Thus, our results suggest that muscle stretch could promote functional recovery from LC-induced muscle injury.
The CSA of myofibers decreased significantly after LC and remained small in the LC-alone group even on the 21st day after LC. This decrease in fiber size could have resulted from a preferential LC-induced loss of type IIb myofibers, which have a large CSA (more than twofold larger than type I or IIa myofibers), as we previously reported [4]. Because torque generation in the muscle depends on the CSA of myofibers, the decreased CSA caused by a loss of type IIb myofibers after LC may be responsible for the decrease in maximal isometric torque. A single bout of muscle stretch applied 1 day after LC contributed to the recovery of CSA within 21 days, resulting in the faster functional recovery, as mentioned above.
Richard–Bulteau et al. reported the positive effects of running exercise on the process of regeneration after muscle injury induced by intramuscular injection of the snake venom notexin [10]. Muscle mass and CSA had fully recovered by the 21st day after injury via the exercise program (1–2 h a day and 5 days a week), which was started on the 4th day after injury. On the other hand, we applied only a single bout of stretch to the muscle 1 day after LC at an intensity of 3 mNm for 15 min, and obtained a significant effect. The intensity of the stretch stimulus was weak and corresponds to about 8% of the maximal isometric torque (approx. 36 mNm) induced by supramaximal transcutaneous electrical stimulation of the ankle extensor muscles. We observed a steeper increase in the passive torque at intensities of >3 mNm for a range of stretch. One possible reason for this steeper increase in torque during stretch may be related to the stretch reflex or the stiffness of tissues other than muscles (tendons, ligaments, and joints) [12, 13]. Although the mechanisms are currently unclear, our findings suggest that a single bout of post-injury muscle stretch at an intensity of 3 mNm, applied in the early stage of recovery, was sufficient to potentiate the regeneration process in the muscle, resulting in accelerated structural and functional recovery from LC-induced muscle injury.
Regeneration of skeletal muscles from injuries occurs due to the myogenic potential of satellite cells lying in close proximity to the myofibers. Adult satellite cells can quickly change their form from quiescent to active in response to injuries or growth signals [14]. Satellite cells can be activated by cytokines and proliferating factors released from inflammatory cells, such as neutrophils and macrophages, which infiltrate into the muscle immediately after injury [15,16,17]. Satellite cells on cultured myofibers have been reported to be activated by mechanical stretch [18], and mechanically activated satellite cells could differentiate into myofibers and proliferate in vitro [19]. In the present study, we applied a mechanical stretch stimulus 1 day after injury. Activated satellite cells are assumed to be differentiating into myoblasts at this time. Thus, mechanical stretch stimulus used in our study could further promote the activation of satellite cells and the differentiation of the activated satellite cells to accelerate the regeneration process in the muscle.
Although the dMHC-ir myofibers appeared on the fifth day after injury in the LC-alone group, a sign of stretch-induced facilitation of the regeneration process was already evident 3 days after injury (i.e., 2 days after stretch), as revealed by the formation of small-sized dMHC-ir myofibers, a marker of newly formed myofibers during muscle regeneration [20]. New myofibers develop from myotube cells formed by the fusion of mononuclear myoblasts. Thus, the stretch-induced activation of satellite cells may have promoted this regeneration process via formation of new myofibers after injury.
The muscle injury model used in the present study has some advantages. First, injury can be induced by LC, not by externally applied myotoxic or inflammatory substances [10, 21, 22]. Second, the model can be produced in vivo without surgical procedures, not in vitro or in situ with invasive procedures. These advantages enable a reflection of the pathophysiological condition of naturally occurring muscle injury in the body.
In our study we used H&E staining and observed histological damage of myofibers and infiltration of mononuclear cells in the entire CSA of the TA that underwent LC. This finding is consistent with that reported in our previous study, i.e., a quarter of the CSA in the TA was occupied by injured myofibers, as revealed using Evans blue dye labeling at 2 days after LC. The maximal isometric torque decreased significantly after LC, to an extent similar to that observed in our previous study (27 vs. 32%, respectively) [4]. These results demonstrate the validity and reproducibility of the rat muscle injury model used in the present study.
References
Proske U, Morgan DL (2001) Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol. doi:10.1111/j.1469-7793.2001.00333.x
McCully KK, Faulkner JA (1985) Injury to skeletal muscle fibers of mice following lengthening contractions. J Appl Physiol 59(1):119–126
Friden J, Lieber RL (1992) Structural and mechanical basis of exercise-induced muscle injury. Med Sci Sports Exerc. doi:10.1249/00005768-199205000-00005
Mori T, Agata N, Itoh Y, Miyazu-Inoue M, Sokabe M, Taguchi T, Kawakami K (2014) Stretch speed-dependent myofiber damage and functional deficits in rat skeletal muscle induced by lengthening contraction. Physiol Rep. doi:10.14814/phy2.12213
Hayashi K, Katanosaka K, Abe M, Yamanaka A, Nosaka K, Mizumura K, Taguchi T (2017) Muscular mechanical hyperalgesia after lengthening contractions in rats depends on stretch velocity and range of motion. Eur J Pain. doi:10.1002/ejp.909
Warren GL, Hayes DA, Lowe DA, Armstrong RB (1993) Mechanical factors in the initiation of eccentric contraction-induced injury in rat soleus muscle. J Physiol. doi:10.1113/jphysiol.1993.sp019645
Mizumura K, Taguchi T (2016) Delayed onset muscle soreness: involvement of neurotrophic factors. J Physiol Sci. doi:10.1007/s12576-015-0397-0
Koh TJ, Peterson JM, Pizza FX, Brooks SV (2003) Passive stretches protect skeletal muscle of adult and old mice from lengthening contraction-induced injury. J Gerontol A Biol Sci Med Sci. doi:10.1093/gerona/58.7.B592
Inoue T, Suzuki S, Hagiwara R, Iwata M, Banno Y, Okita M (2009) Effects of passive stretching on muscle injury and HSP expression during recovery after immobilization in rats. Pathobiology. doi:10.1159/000228901
Richard-Bulteau H, Serrurier B, Crassous B, Banzet S, Peinnequin A, Bigard X, Koulmann N (2008) Recovery of skeletal muscle mass after extensive injury: positive effects of increased contractile activity. Am J Physiol Cell Physiol. doi:10.1152/ajpcell.00355.2007
Chan YS, Hsu KY, Kuo CH, Lee SD, Chen SC, Chen WJ, Ueng SW (2010) Using low-intensity pulsed ultrasound to improve muscle healing after laceration injury: an in vitro and in vivo study. Ultrasound Med Biol. doi:10.1016/j.ultrasmedbio.2010.02.010
Thompson FJ, Browd CR, Carvalho PM, Hsiao J (1996) Velocity-dependent ankle torque in the normal rat. NeuroReport 7(14):2273–2276
De-Doncker L, Picquet F, Petit J, Falempin M (2003) Characterization of spindle afferents in rat soleus muscle using ramp-and-hold and sinusoidal stretches. J Neurophysiol. doi:10.1152/jn.00153.2002
Dumont NA, Bentzinger CF, Sincennes MC, Rudnicki MA (2015) Satellite cells and skeletal muscle regeneration. Compr Physiol. doi:10.1002/cphy.c140068
Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE (1998) HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. doi:10.1006/dbio.1997.8803
Tidball JG (2005) Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. doi:10.1152/ajpregu.00454.2004
Tidball JG, Villalta SA (2010) Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol. doi:10.1152/ajpregu.00735.2009
Wozniak AC, Pilipowicz O, Yablonka-Reuveni Z, Greenway S, Craven S, Scott E, Anderson JE (2003) C-Met expression and mechanical activation of satellite cells on cultured muscle fibers. J Histochem Cytochem. doi:10.1177/002215540305101104
Tatsumi R, Sheehan SM, Iwasaki H, Hattori A, Allen RE (2001) Mechanical stretch induces activation of skeletal muscle satellite cells in vitro. Exp Cell Res. doi:10.1006/excr.2001.5252
Smith HK, Plyley MJ, Rodgers CD, McKee NH (1999) Expression of developmental myosin and morphological characteristics in adult rat skeletal muscle following exercise-induced injury. Eur J Appl Physiol Occup Physiol. doi:10.1007/s004210050562
McNeill Ingham SJ, de Castro Pochini A, Oliveira DA, Garcia Lisboa BC, Beutel A, Valero-Lapchik VB, Ferreira AM, Abdalla RJ, Cohen M, Han SW (2011) Bupivacaine injection leads to muscle force reduction and histologic changes in a murine model. PM&R. doi:10.1016/j.pmrj.2011.05.027
Pinniger GJ, Lavin T, Bakker AJ (2012) Skeletal muscle weakness caused by carrageenan-induced inflammation. Muscle Nerve. doi:10.1002/mus.23318
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research (B) (JSPS KAKENHI Grant numbers JP15H03042 to KK, and JP25282160 and JP16H03202 to TT) and for Challenging Exploratory Research (JP25560254 and JP16K12938 to KK), as well as by the Japan Agency for Medical Research and Development (AMED) Grant 16gm0810010h0502 (to TT) and by funding from the Hori Sciences and Arts Foundation (to TT).
Author information
Authors and Affiliations
Contributions
Author contribution
TM, NA, YI, and MI-M performed the histological analyses and muscle function tests; TM, TT, and KK conceived the study and drafted the manuscript; KM and MS revised the manuscript critically.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Mori, T., Agata, N., Itoh, Y. et al. Post-injury stretch promotes recovery in a rat model of muscle damage induced by lengthening contractions. J Physiol Sci 68, 483–492 (2018). https://doi.org/10.1007/s12576-017-0553-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12576-017-0553-9