Abstract
Disseminated metastasis is associated with a poor prognosis, and its management in the peritoneal or pleural cavity is crucial in the treatment of cancer. Recent studies show that ion and water transporters play important roles in fundamental cellular functions, including the regulation of cell volume that would be involved in the cancer process. Here, we review the evidence for hypotonic treatments of cancer and evaluate the potential of the cellular physiological approach in clinical management. The regulation of extracellular osmolality is a promising method, with several studies demonstrating the cytocidal effects of hypotonic solution on cancer cells. Peritoneal lavage with distilled water (DW) during surgery is reported to improve the survival rate of patients with spontaneously ruptured hepatocellular carcinoma. The in vitro studies included in this review also indicate the cytocidal effects of hypotonic shock on esophageal, gastric, colonic, pancreatic, and liver cancer cells with several unique methods and apparatuses, such as a differential interference contrast microscope connected to a digital video camera, a high-resolution flow cytometer and re-incubation analysis. The in vivo studies demonstrate the safeness of a peritoneal injection of DW into mice and indicate that the development of dissemination nodules can be prevented by the pre-incubation of cancer cells with DW or the peritoneal injection of DW. We also demonstrate that the blockade of Cl− channels/transporters enhances the cytocidal effects of hypotonic shock by inhibiting regulatory volume decrease in various cancer cells. A deeper understanding of molecular mechanisms may lead to the discovery of these cellular physiological approaches as a novel therapeutic strategy for disseminated metastasis.
Similar content being viewed by others
Introduction
Disseminated metastasis is one of the most common forms of recurrence of disease and is associated with a poor prognosis in patients with cancer. Therefore, the management of dissemination in the peritoneal or pleural cavity is crucial in the treatment of cancer [1–4]. However, there are currently few effective treatments for disseminated metastasis, and although limited success with some current treatment methods, such as intraperitoneal chemotherapy, has been reported [5–7], novel strategies for the treatment of disseminated metastasis need to be established to achieve better results.
The roles of ion and water transporters have been examined in cancer cells, and the results of such studies suggest that various cellular physiological approaches have potential as novel therapeutic strategies [8–14]. The regulation of extracellular osmolality is one such promising method, with previous studies having demonstrated the cytocidal effects of hypotonic solution on cancer cells [15–18]. Peritoneal or pleural lavage with distilled water (DW) has been described after surgery for various cancers, with the hypothesis that the hypotonic shock conferred to potential free cancer cells may result in cell lysis and the prevention of peritoneal or pleural seeding.
The aim of this article was to provide a systematic review of current knowledge of hypotonic treatments of cancer. The ultimate objective was to evaluate the potential of the cellular physiological approach, such as regulation of osmolality and ion channels/transporters, in clinical management of cancer.
Cytocidal effect of hypotonic shock in cancer cells: in vitro studies
Several in vitro studies have investigated the cytocidal effects of hypotonic stress on cancer cells [15–18]. An early study in breast cancer cells demonstrated that exposure to water in vitro could generate significant cytotoxic effects after 1 min [15]. Huguet et al. examined the cytocidal activity of DW on colorectal cancer cell lines in culture and reported that the incubation of these cells in DW resulted in cell lysis, with 100% lysis achieved after 14 min of incubation [16]. In an in vitro study, Zhou et al. showed that a 15-min exposure of hepatocellular carcinoma (HCC) cells to DW resulted in complete cell lysis [17]. Fechner et al. found that a 10-min exposure of bladder cancer cells to DW led to significant cytolysis of the cells [18]. The effect of hypotonic shock on the therapeutic efficacy and/or intracellular uptake of chemotherapeutic agents has also been examined. Levin et al. showed that chemotherapeutic agents dissolved in DW were more destructive than chemotherapeutic agents dissolved in saline [19]. Along the same line, Ichinose et al. showed that the growth inhibition of tumor cells after hypotonic cisplatin treatment was significantly greater than that after treatment with saline solution and cisplatin, leading the authors to develop a novel treatment for carcinomatous pleuritis in patients with lung cancer [20].
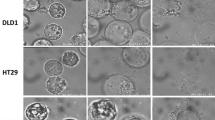
To the contrary, Mercill et al. reported that breast, ovarian, gastric, bladder, and melanoma cell lines were damaged to varying degrees by exposure to DW, but that viable cells persisted in all cases, leading these authors to conclude that hypotonic shock is not an effective method to kill human tumor cells [21]. To determine the osmolarity and incubation time necessary to kill cancer cells in detail, our research group has examined changes in the cellular morphology and volume of cancer cells subjected to hypotonic shock using several unique methods and apparatuses, such as a differential interference contrast microscope connected to a digital video camera and a high-resolution flow cytometer [22–26]. Autoclaved Milli-Q water was used as our DW working solution [22–26]. Video recordings using a digital camera (30 frames/s, 512 × 512 pixels, 10 bits per pixel) demonstrated that hypotonic shock with DW induced swelling and then rupture in esophageal, gastric, colonic, pancreatic, and liver cancer cell lines (Fig. 1) [22–26]. Rupture of cancer cells occurred within 3–10 min after perfusion with DW.
Changes in hepatocellular carcinoma (HCC) cells after exposure to hypotonic stress induced by distilled water (DW). Video recordings were made using a differential interference contrast microscope connected to a digital video camera. HCC cells (Alexander cells) started to swell as soon as they had been exposed to the DW and then continued to swell until they ruptured. Bar: 10.0 μm
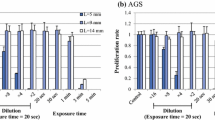
To quantify serial cell volume changes in cancer cells after exposure to hypotonic shock of various osmolarities, our group measured cell volume and cell counts simultaneously after the hypotonic shock using a high-resolution flow cytometer (Cell Lab Quanta (Beckman Coulter, Brea, CA) [22–26]. This flow cytometer is designed to measure the electronic volume by the Coulter principle and is more accurate than the volume detected by forward angle light scattering analysis with a conventional flow cytometer. Once cancer cells were suspended in the respective hypotonic buffer, the cells initially increased in cell volume. Upon exposure of the cells to mild hypotonicity, the cell volume decreased gradually to their pre-hypotonic shock level, suggesting that mild hypotonicity could induce regulatory volume decrease (RVD) in cancer cells (Fig. 2) [27, 28]. When the cells were exposed to severe hypotonicity with DW, the amount of broken fragments of cancer cells increased within 5 min [22–26] (Fig. 2). We also found differences in the cell volume changes caused by hypotonic shock among cell lines, suggesting that cancer cells differ in terms of their resistance to hypotonic shock. To confirm the cytocidal effects of hypotonic shock with DW on cancer cells, we re-incubated these cells after they had been exposed to DW [22–26]. The decrease in the number of cancer cells depended on the duration of exposure to DW in each of cancer cell lines. We also found differences in the cytocidal effects of hypotonic shock with DW among cell lines [22–26]. Taoka et al. also showed that the cytocidal effect of hypotonic shock can be achieved, to varying degrees, in three different bladder cancer cell lines with exposure to DW [29]. In terms of the effects on non-cancerous cells, we previously reported that pancreatic cancer cells were more sensitive to hypotonic shock than fibroblast cells [24].
Cell volume changes in esophageal cancer cells (line KYSE170) after hypotonic shock. The cell volume and the cell counts were simultaneously measured at 1, 5, 10 and 20 min after hypotonic shock at various osmolarities using a high-resolution flow cytometer (Cell Lab Quanta). When the cells were exposed to mild hypotonicity (×1/2 NaCl, i.e., the isotonic NaCl buffer was diluted twofold with DW), the cell volume initially increased and then decreased gradually to the pre-hypotonic shock level, suggesting that mild hypotonicity could induce regulatory volume decrease. When the cells were exposed to DW, the overall cell volume was smaller than the pre-hypotonic shock volume, suggesting that almost all of the cells had broken into fragments. Exposure to isotonic NaCl buffer did not cause any significant changes in cell volume within 20 min (data not shown)
These results of in vitro studies suggest that hypotonic shock could be applied for the treatment of dissemination by using a peritoneal or pleural injection of hypotonic solution.
Cytocidal effect of hypotonic shock in cancer cells: in vivo studies
In vitro studies initially confirmed the cytocidal effects of hypotonic shock on cancer cells, but at that time the effects of hypotonic treatments on the development of dissemination and their safeness in vivo had not been fully evaluated. Using a mouse model of colorectal cancer, Ito et al. reported that all mice who had peritoneal lavage with DW survived, suggesting that DW did not cause fatal damage in vivo [30]. In a subsequent experiment to determine the toxicity of the peritoneal injection of DW in vivo, our group injected 2 ml of DW into the abdominal cavities of mice for 3 days [31]. Macroscopic and microscopic examinations revealed that the peritoneal injection of DW did not severely damage the abdominal organs of mice, suggesting its safeness in an in vivo setting [31].
Several studies have investigated the efficacy of osmotic lysis on cancer cells by using an in vivo model. Ito et al. established a reproducible model of tumor spill by using mouse colorectal cancer cells to reproduce water or saline lavage during laparotomy and showed that the water lavage resulted in superior clinical outcomes with a decrease in tumor burden and concomitant improvement in survival [30]. Huguet et al. investigated the optimal method for peritoneal lavage with DW during colorectal cancer surgery using an in vivo model [16]. To the contrary, Morris et al. showed that intraperitoneal lavage with DW did not provide protection against the establishment of xenografts after the intraperitoneal injection of human ovarian cancer cells in the nude mouse model [32]. Also using an in vivo model, our group determined the therapeutic effects of a peritoneal injection of DW for the treatment of peritoneal dissemination from gastric cancer [31]. Gastric cancer cells pre-incubated with DW for 20 min in vitro were intraperitoneally injected into nude mice and the development of dissemination nodules analyzed [31]. We found that the total number, weight, and volume of the dissemination nodules were significantly decreased by pre-incubation of the gastric cancer cells in DW [31]. We obtained similar results using DW-treated colorectal cancer cells in an in vivo model [25]. Then, to determine whether the peritoneal injection of DW inhibited the establishment of peritoneal dissemination, we injected DW into the abdominal cavity for 3 days into nude mice after a peritoneal injection of gastric cancer cells [31]. The results showed that the total volume of dissemination nodules was significantly lower in DW-injected mice than in NaCl-injected mice [31]. Zhou et al. also injected DW-treated HCC cells intraperitoneally into nude mice and showed that the formation of tumor nodules was completely prevented and that survival time was prolonged [17]. Further, they examined whether peritoneal injection of DW at specific time points inhibited the establishment of disseminated peritoneal nodules and found that peritoneal administration of DW significantly inhibited the establishment of peritoneal HCC metastases within 48 h after the cells were injected [17]. Taken together, these findings indicate that the development of dissemination nodules in vivo can be prevented by the pre-incubation of cancer cells with DW or peritoneal injection of DW.
The effect of fluid osmolarity on chemotherapy administered into the peritoneal and the pleural cavities in animal experimental models has also been determined [33, 34]. Kondo et al. showed that the uptake of cisplatin in vivo by tumor cells was significantly greater in hypotonic solution than in isotonic or hypertonic solution after intraperitonial administration and that the survival of mice given the cisplatin in hypotonic solution was significantly prolonged [33]. Comparison of the pharmacokinetics of intraperitoneal and intrapleural hypotonic cisplatin under the same in vivo experimental conditions revealed that the amount of platinum taken up by tumor cells was significantly greater in the pleural cavity than in the peritoneal cavity [34]. These results demonstrate that the therapeutic efficacy of intraperitoneal and intrapleural injection of cisplatin in vivo is augmented when the drug is administered in hypotonic solution.
Effect of hypotonic shock in cancer: clinical studies
Disseminated metastasis is considered to be caused by free peritoneal or pleural cancer cells exfoliated from serosally invasive tumors. Exfoliated cancer cells from the primary tumor may be viable and tumorigenic; therefore, effective peritoneal or pleural lavage is clinically crucial at the time of the initial surgery. Whiteside et al. investigated the frequency and pattern of intra-operative peritoneal lavage amongst general surgeons and found that 36% of surgeons used water lavage during intra-abdominal cancer surgery [35]. Several studies have indicated the efficacy of peritoneal lavage with DW during surgery for patients with spontaneously ruptured HCC [17, 36, 37] (Table 1); specifically, these studies showed that DW lavage during liver resection improved long-term outcomes in patients with spontaneously ruptured HCC. Thus, the clinical use of DW lavage during surgery for cancer would appear to retard the tumor recurrence and further improve the survival rate with minimal cost.
The results of several studies indicate the efficacy of the administration of hypotonic intraperitoneal or intrapleural cisplatin during surgery [38–42]. In a randomized trial, Ichinose et al. reported that intra-operative intrapleural treatment with hypotonic cisplatin solution effectively controlled malignant pleural effusion and/or pleural dissemination found at thoracotomy in patients with non-small-cell lung cancer [38]. They also found that the use of intra-operative intrapleural treatment with hypotonic cisplatin in their resected patients with a positive intrapleural lavage cytology finding significantly decreased the occurrence of malignant effusion and/or pleural dissemination after the operation [39]. Tsujitani et al. performed an osmolarity reduction and dose escalation trial for the use of hypotonic intraperitoneal cisplatin treatment in patients with gastric cancer and showed that this therapeutic strategy did not increase the plasma level of platinum at a dose of 70 mg/m2 and was well tolerated [40]. Katano et al. also investigated the pharmacokinetics of intra-operative intrapleural cisplatin chemotherapy at various osmolarities in patients with esophageal cancer and reported that intra-operative intrapleural hypotonic treatment with cisplatin was tolerable [41]. A multi-institutional phase II trial of intrapleural hypotonic cisplatin treatment instilled through a chest tube for malignant pleural effusion in 80 patients with non-small-cell lung cancer revealed that the toxicity of this hypotonic cisplatin treatment was acceptable and that 34 and 49% of patients achieved complete response and partial response, respectively [42]. These results suggest that intraperitoneal or intrapleural administration of chemotherapeutic agents in hypotonic solution can be considered to be both a feasible and effective strategy for the prevention and/or treatment of cancer dissemination (Table 1).
Cellular physiological approach to enhance the efficacy of hypotonic shock
Although the in vivo studies of our group confirmed the efficacy of the peritoneal injection of DW for the prevention of peritoneal dissemination, its inhibitory effects appeared to be weaker than those observed in the DW pre-incubation model [31]. One explanation may be an increase in the osmolarity of extracellular fluids after DW injection caused by the contamination of existing intraperitoneal secretions and many types of disrupted cells. We previously reported that very severe hypotonicity is required to disrupt cancer cells into fragments and that the osmolarity of the fluid collected after peritoneal lavage with DW injection during surgery for gastric cancer is approximately 50 mOsm/kg H2O due to the contamination of disrupted cells [23]. Huguet et al. have also reported that contamination of lavage water by peritoneal secretions produces a resultant solution with an osmolality of 50 mM [16]. These results indicate that the hypotonic solutions used in peritoneal injections should be modified to enhance their inhibitory effects.
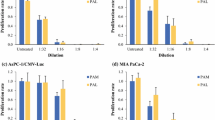
Although the investigation of the cellular physiological mechanism is crucial to increasing the cell swelling induced by hypotonic shock and to enhancing the cytocidal effects, little data are available on the specific mechanism by which the rupture of cancer cells occurs. Our group has attempted to regulate the hypotonicity-induced cell volume change that results from cellular physiological factors, such as changes in ion channels/transporters, by focusing specifically on the control of RVD. RVD occurs after hypotonicity-induced cell swelling, and has been attributed to the activation of ion channels and transporters, which in turn causes K+, Cl−, and H2O effluxes, ultimately leading to cell shrinkage (Fig. 3) [27, 28]. In our studies we challenged the cells with 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB), a Cl− channel blocker, to increase cell volume by inhibiting RVD (Fig. 4) [22–26]. To investigate whether NPPB affects the cell volume changes of cancer cells after hypotonic shock, we measured the cell volume after exposure to the hypotonic buffer containing NPPB using the Cell Lab Quanta flow cytometer [22–26]. We found that NPPB enhanced cell swelling and drastically slowed the time course of the decrease in cell volume (RVD) following the cell swelling induced by hypotonic shock in esophageal, gastric, colonic, pancreatic, and liver cancer cell lines, suggesting that these effects were caused by the inhibition of RVD [22–26].
To investigate whether NPPB enhances the cytocidal effects of hypotonic stress on cancer cells, we re-incubated the cells following their exposure to hypotonic buffer containing NPPB and found that NPPB enhanced the cytocidal effects of hypotonic shock on esophageal, gastric, colonic, pancreatic, and liver cancer cells [22–26]. Our results from gastric cancer cell lines revealed that treatment with NPPB increased cell volume by inhibiting RVD and enhanced the cytocidal effects of the hypotonic solution in the MKN45 and Kato-III cells, whereas no such effects were observed in the MKN28 cells [23]. Interestingly, treatment of the MKN28 cells with R(+)-[(dihydroindenyl) oxy] alkanoic acid, a blocker of the K+/Cl− cotransporter, inhibited RVD and enhanced the cytocidal effects of hypotonic shock [23], hinting at differences in the presence of cell volume mediators among various cell lines and the heterogeneity of cancer cells. Furthermore, RVD was shown to be inhibited by quinine hydrochloride (Quin) or mercury chloride II (Hg) through the blockade of potassium ion or water transport. We challenged the HCC cells with Quin or Hg and found that they enhanced the cytocidal effects of hypotonic shock via the inhibition of RVD [26].
These findings suggest that the regulation of ion transport enhances the cytocidal effects of hypotonic shock on cancer cells. A deeper understanding of ion transport mechanisms in cancer cells during hypotonic shock could lead to the development of novel therapeutic strategies.
Conclusions
This review reports on current knowledge on hypotonic treatments of cancer. The results of the studies discussed here clearly support the efficacy of pleural or peritoneal lavage with DW during surgery. Further, the development of a more specific ion channel blocker or novel small interfering RNA delivery system in vivo will make it possible to enhance the effects of the peritoneal or pleural injection of DW in the treatment of cancer dissemination. A deeper understanding of the underlying molecular mechanisms may lead the application of these cellular physiological approaches, such as the regulation of extracellular osmolality and ion transport, as a novel therapeutic strategy for cancer.
References
Rajdev L (2010) Treatment options for surgically resectable gastric cancer. Curr Treat Options Oncol 11:14–23
Lim L, Michael M, Mann GB, Leong T (2005) Adjuvant therapy in gastric cancer. J Clin Oncol 23:6220–6232
Van-den-Broeck A, Sergeant G, Ectors N, Van-Steenbergen W, Aerts R, Topal B (2009) Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol 35:600–604
Lemmens VE, Klaver YL, Verwaal VJ, Rutten HJ, Coebergh JW, de Hingh IH (2011) Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: a population-based study. Int J Cancer 128:2717–2725
Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ (2009) Gastric cancer. Lancet 374:477–490
Emoto S, Yamaguchi H, Kishikawa J, Yamashita H, Ishigami H, Kitayama J (2012) Antitumor effect and pharmacokinetics of intraperitoneal NK105, a nanomicellar paclitaxel formulation for peritoneal dissemination. Cancer Sci 103:1304–1310
Takahara N, Isayama H, Nakai Y, Sasaki T, Ishigami H, Yamashita H, Yamaguchi H, Hamada T, Uchino R, Mizuno S, Miyabayashi K, Mohri D, Kawakubo K, Kogure H, Yamamoto N, Sasahira N, Hirano K, Ijichi H, Tateishi K, Tada M, Kitayama J, Watanabe T, Koike K (2014) Intravenous and intraperitoneal paclitaxel with S-1 for refractory pancreatic cancer with malignant ascites: an interim analysis. J Gastrointest Cancer 45:307–311
Fraser SP, Pardo LA (2008) Ion channels: functional expression and therapeutic potential in cancer. Colloquium on ion channels and cancer. EMBO Rep 9:512–515
Pedersen SF, Stock C (2013) Ion channels and transporters in cancer: pathophysiology, regulation, and clinical potential. Cancer Res 73:1658–1661
Schönherr R (2005) Clinical relevance of ion channels for diagnosis and therapy of cancer. J Membr Biol 205:175–184
Miyazaki H, Marunaka Y (2015) The molecular mechanism of intracellular Cl− function in gastric cancer invasion and metastasis by regulating expression of cell adhesion molecules. J Physiol Sci 65[Suppl 1]:72
Takada T, Takata K, Ashihara E (2016) Inhibition of monocarboxylate transporter 1 suppresses the proliferation of glioblastoma stem cells. J Physiol Sci 66:387–396
Marunaka Y (2016) Cl− and H+ as mediators of biofunction and biodysfunction in health and disease. J Physiol Sci 66[Suppl 1]:4
Miyazaki H, Ue T, Tanaka S, Nakayama Y, Marunaka Y (2016) The molecular mechanism of intracellular Cl− in cancer progression by regulating Src kinase signal cascades. J Physiol Sci 66[Suppl 1]:159
Park KG, Chetty U, Scott W, Miller W (1991) The activity of locally applied cytotoxics to breast cancer cells in vitro. Ann R Coll Surg Engl 73:96–99
Huguet EL, Keeling NJ (2004) Distilled water peritoneal lavage after colorectal cancer surgery. Dis Colon Rectum 47:2114–2119
Zhou SJ, Zhang EL, Liang BY, Zhang ZY, Chen XP, Huang ZY (2015) Distilled water lavage during surgery improves long-term outcomes of patients with ruptured hepatocellular carcinoma. J Gastrointest Surg 19:1262–1270
Fechner G, Pocha K, Schmidt D, Muller SC (2006) Reducing recurrence and costs in superficial bladder cancer: preclinical evaluation of osmotic cytolysis by distilled water vs. mitomycin. Int J Clin Pract 60:1178–1180
Levin DR, Moskovitz B (1986) Distilled water versus chemotherapeutic agents for transitional bladder carcinoma. Eur Urol 12:418–421
Ichinose Y, Hara N, Ohta M, Asoh H, Yano T, Maeda K, Yagawa K (1993) Hypotonic cisplatin treatment for carcinomatous pleuritis found at thoracotomy in patients with lung cancer. In vitro experiments and preliminary clinical results. J Thorac Cardiovasc Surg 10:1041–1046
Mercill DB, Jones NR, Harbell JW (1985) Human tumor cell destruction by distilled water. An in vitro evaluation. Cancer 55:2779–2782
Kosuga T, Shiozaki A, Ichikawa D, Fujiwara H, Komatsu S, Iitaka D, Tsujiura M, Morimura R, Takeshita H, Nagata H, Okamoto K, Nakahari T, Marunaka Y, Otsuji E (2011) Pleural lavage with distilled water during surgery for esophageal squamous cell carcinoma. Oncol Rep 26:577–586
Iitaka D, Shiozaki A, Ichikawa D, Kosuga T, Komatsu S, Okamoto K, Fujiwara H, Ishii H, Nakahari T, Marunaka Y, Otsuji E (2012) Blockade of chloride ion transport enhances the cytocidal effect of hypotonic solution in gastric cancer cells. J Surg Res 176:524–534
Nako Y, Shiozaki A, Ichikawa D, Komatsu S, Konishi H, Iitaka D, Ishii H, Ikoma H, Kubota T, Fujiwara H, Okamoto K, Ochiai T, Nakahari T, Marunaka Y, Otsuji E (2012) Enhancement of the cytocidal effects of hypotonic solution using a chloride channel blocker in pancreatic cancer cells. Pancreatology 12:440–448
Takemoto K, Shiozaki A, Ichikawa D, Komatsu S, Konishi H, Nako Y, Murayama Y, Kuriu Y, Nakanishi M, Fujiwara H, Okamoto K, Sakakura C, Nakahari T, Marunaka Y, Otuji E (2015) Evaluation of the efficacy of peritoneal lavage with distilled water in colorectal cancer surgery: in vitro and in vivo study. J Gastroenterol 50:287–297
Kudou M, Shiozaki A, Kosuga T, Ichikawa D, Konishi H, Morimura R, Komatsu S, Ikoma H, Fujiwara H, Okamoto K, Hosogi S, Nakahari T, Marunaka Y, Otsuji E (2016) Inhibition of regulatory volume decrease enhances the cytocidal effect of hypotonic shock in hepatocellular carcinoma. J Cancer 7:1524–1533
Caplanusi A, Kim KJ, Lariviere E, Van Driessche W, Jans D (2006) Swelling-activated K+ efflux and regulatory volume decrease efficiency in human bronchial epithelial cells. J Membr Biol 214:33–41
Miyazaki H, Shiozaki A, Niisato N, Marunaka Y (2007) Physiological significance of hypotonicity-induced regulatory volume decrease: reduction in intracellular Cl− concentration acting as an intracellular signaling. Am J Physiol Renal Physiol 292:F1411–1417
Taoka R, Williams SB, Ho PL, Kamat AM (2015) In-vitro cytocidal effect of water on bladder cancer cells: the potential role for intraperitoneal lavage during radical cystectomy. Can Urol Assoc J 9:E109–113
Ito F, Camoriano M, Seshadri M, Evans SS, Kane JM 3rd, Skitzki JJ (2011) Water: a simple solution for tumor spillage. Ann Surg Oncol 18:2357–2363
Shiozaki A, Ichikawa D, Takemoto K, Nako Y, Nakashima S, Shimizu H, Kitagawa M, Kosuga T, Konishi H, Komatsu S, Fujiwara H, Okamoto K, Marunaka Y, Otsuji E (2014) Efficacy of a hypotonic treatment for peritoneal dissemination from gastric cancer cells: an in vivo evaluation. Biomed Res Int 2014:707089
Morris PC, Scholten V (1996) Osmotic lysis of tumor spill in ovarian cancer: a murine model. Am J Obstet Gynecol 175:1489–1492
Kondo A, Maeta M, Oka A, Tsujitani S, Ikeguchi M, Kaibara N (1996) Hypotonic intraperitoneal cisplatin chemotherapy for peritoneal carcinomatosis in mice. Br J Cancer 73:1166–1170
Katano K, Tsujitani S, Oka S, Saito H, Gomyo Y, Kondo A, Ikeguchi M, Maeta M, Kaibara N (2000) Pharmacokinetics of hypotonic cisplatin chemotherapy administered into the peritoneal and the pleural cavities in experimental model. Anticancer Res 20:1603–1607
Whiteside OJ, Tytherleigh MG, Thrush S, Farouk R, Galland RB (2005) Intra-operative peritoneal lavage—who does it and why? Ann R Coll Surg Engl 87:255–258
Lin CH, Hsieh HF, Yu JC, Chen TW, Yu CY, Hsieh CB (2006) Peritoneal lavage with distilled water during liver resection in patients with spontaneously ruptured hepatocellular carcinomas. J Surg Oncol 94:255–256
Chang YM, Hsu KF, Yu JC, Chan DC, Chen CJ, Chen TW, Hsieh CB, Hsieh HF (2013) Distilled water peritoneal lavage in patients with rupture hepatocellular carcinoma. Hepatogastroenterology 60:140–143
Ichinose Y, Yano T, Asoh H, Yokoyama H, Fukuyama Y, Miyagi J, Kuninaka S, Terazaki Y (1997) Intraoperative intrapleural hypotonic cisplatin treatment for carcinomatous pleuritis. J Surg Oncol 66:196–200
Ichinose Y, Tsuchiya R, Koike T, Yasumitsu T, Nakamura K, Tada H, Yoshimura H, Mitsudomi T, Nakagawa K, Yokoi K, Kato H (2002) A prematurely terminated phase III trial of intraoperative intrapleural hypotonic cisplatin treatment in patients with resected non-small cell lung cancer with positive pleural lavage cytology: the incidence of carcinomatous pleuritis after surgical intervention. J Thorac Cardiovasc Surg 123:695–699
Tsujitani S, Fukuda K, Saito H, Kondo A, Ikeguchi M, Maeta M, Kaibara N (2002) The administration of hypotonic intraperitoneal cisplatin during operation as a treatment for the peritoneal dissemination of gastric cancer. Surgery 131:S98–S104
Katano K, Tsujitani S, Maeta M, Fukuda K, Oka S, Hisamitsu K, Ikeguchi M, Kaibara N (2001) Pharmacokinetics of intraoperative intrapleural cisplatin chemotherapy of various osmolarities in cases of esophageal cancer. Oncol Rep 8:605–609
Seto T, Ushijima S, Yamamoto H, Ito K, Araki J, Inoue Y, Semba H, Ichinose Y, Thoracic Oncology Group (2006) Intrapleural hypotonic cisplatin treatment for malignant pleural effusion in 80 patients with non-small-cell lung cancer: a multi-institutional phase II trial. Br J Cancer 95:717–721
Acknowledgements
The authors would like to thank Dr. Michihiro Kudou for technical assistance with the experiments.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Shiozaki, A., Ichikawa, D., Kosuga, T. et al. Regulation of osmolality for cancer treatment. J Physiol Sci 67, 353–360 (2017). https://doi.org/10.1007/s12576-017-0528-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12576-017-0528-x