Abstract
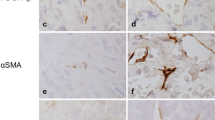
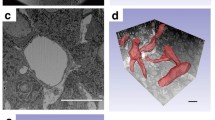
Pericytes are perivascular cells associated with capillaries. We previously demonstrated that pericytes, identified by desmin immunohistochemistry, produce type I and III collagens in the anterior pituitary gland of adult rats. In addition, we recently used desmin immunoelectron microscopy to characterize a novel type of perivascular cell, dubbed a desmin-immunopositive perivascular cell, in the anterior pituitary. These two types of perivascular cells differ in fine structure. The present study attempted to characterize the morphological features of pituitary pericytes and novel desmin-immunopositive perivascular cells during postnatal development, in particular their role in collagen synthesis. Desmin immunostaining revealed numerous perivascular cells at postnatal day 5 (P5) and P10. Transmission electron microscopy showed differences in the fine structure of the two cell types, starting at P5. Pericytes had well-developed rough endoplasmic reticulum and Golgi apparatus at P5 and P10. The novel desmin-immunopositive perivascular cells exhibited dilated cisternae of rough endoplasmic reticulum at P5–P30. In addition, during early postnatal development in the gland, a number of type I and III collagen-expressing cells were observed, as were high expression levels of these collagen mRNAs. We conclude that pituitary pericytes and novel desmin-immunopositive perivascular cells contain well-developed cell organelles and that they actively synthesize collagens during the early postnatal period.






Similar content being viewed by others
References
Díaz-Flores L, Gutiérrez R, Varela H, Rancel N, Valladares F (1991) Microvascular pericytes: a review of their morphological and functional characteristics. Histol Histopathol 6:269–286
Douglas T, Heinemann S, Bierbaum S, Scharnweber D, Worch H (2006) Fibrillogenesis of collagen types I, II, and III with small leucine-rich proteoglycans decorin and biglycan. Biomacromolecules 7:2388–2393
Fujiwara K, Kikuchi M, Takigami S, Kouki T, Yashiro T (2007) Expression of retinaldehyde dehydrogenase 1 in the anterior pituitary glands of adult rats. Cell Tissue Res 329:321–327
Fujiwara K, Jindatip D, Kikuchi M, Yashiro T (2010) In situ hybridization reveals that type I and III collagens are produced by pericytes in the anterior pituitary gland of rats. Cell Tissue Res 342:491–495
Gon G, Shirasawa N, Ishikawa H (1987) Appearance of the cyst- or ductule-like structures and their role in the restoration of the rat pituitary autograft. Anat Rec 217:371–378
González B, Solano-Agama Mdel C, González Del Pliego M, Mendoza-Garrido ME (2004) Differences in cell migration of cultured pituitary cells from infantile and adult rats: participation of the extracellular matrix and epidermal growth factor. Int J Dev Neurosci 22:231–239
Horiguchi K, Kikuchi M, Kusumoto K, Fujiwara K, Kouki T, Kawanishi K, Yashiro T (2010) Living-cell imaging of transgenic rat anterior pituitary cells in primary culture reveals novel characteristics of folliculo-stellate cells. J Endocrinol 204:115–123
Horiguchi K, Fujiwara K, Ilmiawati C, Kikuchi M, Tsukada T, Kouki T, Yashiro T (2011) Caveolin 3-mediated integrin β1 signaling is required for the proliferation of folliculostellate cells in rat anterior pituitary gland under the influence of extracellular matrix. J Endocrinol 210:29–36
Horiguchi K, Syaidah R, Fujiwara K, Tsukada T, Ramadhani D, Jindatip D, Kikuchi M, Yashiro T (2013) Expression of small leucine-rich proteoglycans in rat anterior pituitary gland. Cell Tissue Res 351:207–212
Ilmiawati C, Horiguchi K, Fujiwara K, Yashiro T (2012) Matrix metalloproteinase-9 expression in folliculostellate cells of rat anterior pituitary gland. J Endocrinol 212:363–370
Inoue K, Couch EF, Takano K, Ogawa S (1999) The structure and function of folliculo-stellate cells in the anterior pituitary gland. Arch Histol Cytol 62:205–218
Jindatip D, Fujiwara K, Kouki T, Yashiro T (2012) Transmission and scanning electron microscopy study of the characteristics and morphology of pericytes and novel desmin-immunopositive perivascular cells before and after castration in rat anterior pituitary gland. Anat Sci Int 87:165–173
Kalamajski S, Oldberg A (2010) The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol 29:248–253
Paez-Pereda M, Kuchenbauer F, Arzt E, Stalla GK (2005) Regulation of pituitary hormones and cell proliferation by components of the extracellular matrix. Braz J Med Biol Res 38:1487–1494
Shirasawa N, Yoshimura F (1982) Immunohistochemical and electron microscopical studies of mitotic adenohypophysial cells in different ages of rats. Anat Embryol (Berl) 165:51–61
Shirasawa N, Mabuchi Y, Sakuma E et al (2004) Intercellular communication within the rat anterior pituitary gland: X. Immunohistocytochemistry of S-100 and connexin 43 of folliculo-stellate cells in the rat anterior pituitary gland. Anat Rec A Discov Mol Cell Evol Biol 278:462–473
Soji T, Herbert DC (1989) Intercellular communication between rat anterior pituitary cells. Anat Rec 224:523–533
Soji T, Shirasawa N, Kurono C, Yashiro T, Herbert DC (1994) Immunohistochemical study of the post-natal development of the folliculo-stellate cells in the rat anterior pituitary gland. Tissue Cell 26:1–8
Verhoeven D, Buyssens N (1988) Desmin-positive stellate cells associated with angiogenesis in a tumour and non-tumour system. Virchows Arch B Cell Pathol Incl Mol Pathol 54:263–272
Acknowledgments
We are grateful Megumi Yatabe for her suggestions on transmission electron microscopic procedure. We also thank David Kipler of Supernatant Communications for revising the language of the manuscript. This work was supported in part by the Research Award to JMU Graduate student, promotional funds for the Keirin Race of the Japan Keirin Association, and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan to T.Y. (22590192).
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
12565_2013_180_MOESM1_ESM.ppt
Supplementary Fig. 1 Triple fluorescence labeling of in situ hybridization for col1a1 mRNA with histochemistry for desmin and isolectin B4 in the anterior pituitary gland of adult rats. The in situ hybridization signal of col1a1 was visualized with a HNPP Fluorescent Detection Kit (Roche Diagnostics) (a, e). After in situ hybridization, pericytes (b) and novel desmin-immunopositive perivascular cells (f) were detected by anti-desmin (diluted 1:1200, Abcam) and Alexa Fluor 488-conjugated anti-rabbit IgG (diluted 1:200, Life Technologies). Then, sections were treated with biotinylated isolectin B4 (diluted 1:25, Vector Laboratories) and Alexa Fluor 633-conjugated streptavidin (diluted 1:400, Life Technologies) to detect endothelial cells (c, g). Col1a1 mRNA was expressed in both a pericyte (d; arrow) and a novel desmin-immunopositive perivascular cell (h; arrowhead). Nuclei were counterstained by Vectashield Mounting Medium containing 4,6-diamidino-2-phenylindole (DAPI; Vector Laboratories). Fluorescence images were created with confocal laser microscopy. Emissions were recorded by photomultipliers using the in situ hybridization signal (red pseudocolor), Alexa Fluor 488 (green pseudocolor), Alexa Fluor 633 (white pseudocolor), and DAPI (blue pseudocolor). ca; capillary. Bar 10 μm (PPT 1410 kb)
Rights and permissions
About this article
Cite this article
Jindatip, D., Fujiwara, K., Horiguchi, K. et al. Changes in fine structure of pericytes and novel desmin-immunopositive perivascular cells during postnatal development in rat anterior pituitary gland. Anat Sci Int 88, 196–203 (2013). https://doi.org/10.1007/s12565-013-0180-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-013-0180-3