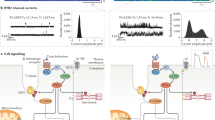
Abstract
Heart failure (HF) is a complication of multiple cardiac diseases and is characterized by impaired contractile and electric function. Patients with HF are not only limited by reduced contractile function but are also prone to life-threatening ventricular arrhythmias. HF itself leads to remodeling of ion channels, gap junctions, and intracellular calcium handling abnormalities that in combination with structural remodeling, e.g., fibrosis, produce a substrate for an arrhythmogenic disorders. Not only ventricular life-threatening arrhythmias contribute to increased morbidity and mortality but also atrial arrhythmias, especially atrial fibrillation (AF), are common in HF patients and contribute to morbidity and mortality. The distinct ion channel remodeling processes in HF and in channelopathies associated with HF will be discussed. Further basic research and clinical studies are needed to identify underlying molecular pathways of HF pathophysiology to provide the basis for improved patient care and individualized therapy based on individualized ion channel composition and remodeling.





Similar content being viewed by others
References
Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, Ellinor PT, Gollob M, Hamilton R, Hershberger RE, Judge DP, Le Marec H, McKenna WJ, Schulze-Bahr E, Semsarian C, Towbin JA, Watkins H, Wilde A, Wolpert C, Zipes DP (2011) HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies. This document was developed as a partnership between the Heart Rhythm Society (HRS) and the European heart rhythm association (EHRA). Europace 13(8):S. 1077–S. 1109
Ai X, Pogwizd SM (2005) Connexin 43 downregulation and dephosphorylation in nonischemic heart failure is associated with enhanced colocalized protein phosphatase type 2A. Circ Res 96(1):S. 54–S. 63
Akar FG, Rosenbaum DS (2003) Transmural electrophysiological heterogeneities underlying arrhythmogenesis in heart failure. Circ Res 93(7):S. 638–S. 645
Akar FG, Spragg DD, Tunin RS, Kass DA, Tomaselli GF (2004) Mechanisms underlying conduction slowing and arrhythmogenesis in nonischemic dilated cardiomyopathy. Circ Res 95(7):S. 717–S. 725
Akhirome E, Jay PY (2015) Rhythm genes sing more than one tune: noncanonical functions of cardiac ion channels. Circ Arrhythm Electrophysiol 8(2):261–262
Alders M, Koopmann TT, Christiaans I, Postema PG, Beekman L, Tanck MW, Zeppenfeld K, Loh P, Koch KT, Demolombe S, Mannens MM, Bezzina CR, Wilde AA (2009) Haplotype-sharing analysis implicates chromosome 7q36 harboring DPP6 in familial idiopathic ventricular fibrillation. Am J Hum Genet 84(4):S. 468–S. 476
Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Hlatky MA, Granger CB, Hammill SC, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL (2017) 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Rhythm Society. Heart Rhythm 2017. https://doi.org/10.1010/j.jacc.2017.10.054
Antzelevitch C, Pollevick GD, Cordeiro JM, Casis O, Sanguinetti MC, Aizawa Y, Guerchicoff A, Pfeiffer R, Oliva A, Wollnik B, Gelber P, Bonaros EP, Burashnikov E, Wu Y, Sargent JD, Schickel S, Oberheiden R, Bhatia A, Hsu LF, Haïssaguerre M, Schimpf R, Borggrefe M, Wolpert C (2007) Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation 115(4):S. 442–S. 449
Baumgarten CM, Clemo HF (2003) Swelling-activated chloride channels in cardiac physiology and pathophysiology. Prog Biophys Mol Biol 82(1–3):S. 25–S. 42
Bellocq C, van Ginneken AC, Bezzina CR, Alders M, Escande D, Mannens MM, Baró I, Wilde AA (2004) Mutation in the KCNQ1 gene leading to the short QT-interval syndrome. Circulation 109: 2394–2397
Bers DM, Lederer WJ (2008) Excitation-contraction coupling and cardiac contractile force, 2nd edn. Springer, Dordrecht
Bers DM, Guo T (2005) Calcium signaling in cardiac ventricular myocytes. Ann N Y Acad Sci 1047:S. 86–S. 98
Beuckelmann DJ, Erdmann E (1992) Ca2+-currents and intracellular Ca2+i-transients in single ventricular myocytes isolated from terminally failing human myocardium. Basic Res Cardiol 87(Suppl 1):S. 235–S. 243
Beuckelmann DJ, Näbauer M, Erdmann E (1993) Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res 73(2):S. 379–S. 385
Bezzina CR, Lahrouchi N, Priori SG (2015) Genetics of sudden cardiac death. Circ Res 116(12):S. 1919–S. 1936
Bhuiyan ZA, van den Berg MP, van Tintelen JP, Bink-Boelkens MT, Wiesfeld AC, Alders M, Postma AV, van Langen I, Mannens MM, Wilde AA (2007) Expanding spectrum of human RYR2-related disease new electrocardiographic, structural, and genetic features. Circulation 116(14):S. 1569–S. 1576
Brado J, Dechant MJ, Menza M, Komancsek A, Lang CN, Bugger H, Foell D, Jung BA, Stiller B, Bode C, Odening KE (2017) Phase-contrast magnet resonance imaging reveals regional, transmural, and base-to-apex dispersion of mechanical dysfunction in patients with long QT syndrome. Heart Rhythm 14(9):S. 1388–S. 1397
Brugada P, Brugada J (1992) Right bundle branch block, persistent ST segment elevation and sudden cardiac death a distinct clinical and electrocardiographic syndrome. J Am Coll Cardiol 20(6):S. 1391–S. 1396
Brugada R, Hong K, Dumaine R, Cordeiro J, Gaita F, Borggrefe M, Menendez TM, Brugada J, Pollevick GD, Wolpert C, Burashnikov E, Matsuo K, Wu YS, Guerchicoff A, Bianchi F, Giustetto C, Schimpf R, Brugada P, Antzelevitch C (2004) Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation 109(1):S. 30–S. 35
Caldwell JL, Smith CE, Taylor RF, Kitmitto A, Eisner DA, Dibb KM, Trafford AW (2014) Dependence of cardiac transverse tubules on the BAR domain protein amphiphysin II (BIN-1). Circ Res 115(12):986–996
Chahal AA, Somers VK (2016) Ion channel remodeling-a potential mechanism linking sleep apnea and sudden cardiac death. J Am Heart Assoc 5(8):e004195
Chen X (2002) L-type Ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ Res 91(6):S. 517–S. 524
Cingolani E, Ramirez Correa GA, Kizana E, Murata M, Cho HC, Marbán E (2007) Gene therapy to inhibit the calcium channel beta subunit physiological consequences and pathophysiological effects in models of cardiac hypertrophy. Circ Res 101(2):S. 166–S. 175
Clemo HF, Stambler BS, Baumgarten CM (1999) Swelling-activated chloride current is persistently activated in ventricular myocytes from dogs with tachycardia-induced congestive heart failure. Circ Res 84(2):S. 157–S. 165
Curran J, Mohler P (2015) Alternative paradigms for ion channelopathies: disorders of ion channel membrane trafficking and posttranslational modification. Annu Rev Physiol 77:S. 505–S. 524
Dobrev D, Ravens U (2003) Remodeling of cardiomyocyte ion channels in human atrial fibrillation. Basic Res Cardiol 98(3):S. 137–S. 148
Duan D (2009) Phenomics of cardiac chloride channels: the systematic study of chloride channel function in the heart. J Physiol 587(Pt 10):S. 2163–S. 2177
Duhme N, Schweizer PA, Thomas D, Becker R, Schröter J, Barends TR, Schlichting I, Draguhn A, Bruehl C, Katus HA, Koenen M (2013) Altered HCN4 channel C-linker interaction is associated with familial tachycardia-bradycardia syndrome and atrial fibrillation. Eur Heart J 34(35):2768–2775
Dupont E, Matsushita T, Kaba RA, Vozzi C, Coppen SR, Khan N, Kaprielian R, Yacoub MH, Severs NJ (2001) Altered connexin expression in human congestive heart failure. J Mol Cell Cardiol 33(2):S. 359–S. 371
Ehrlich JR, Nattel S, Hohnloser SH (2002) Atrial fibrillation and congestive heart failure. Specific considerations at the intersection of two common and important cardiac disease sets. J Cardiovasc Electrophysiol 13(4):S. 399–S. 405
Fu Y, Hong T (2016) BIN1 regulates dynamic t-tubule membrane. Biochim Biophys Acta 1863(7 Pt B):S. 1839–S. 1847
Garcia-Elias A, Benito B (2018) Ion Channel disorders and sudden cardiac death. Int J Mol Sci 19(3)
Gourraud J-B, Le Scouarnec S, Sacher F, Chatel S, Derval N, Portero V, Chavernac P, Sandoval JE, Mabo P, Redon R, Schott J-J, Le Marec H, Haïssaguerre M, Probst V (2013) Identification of large families in early repolarization syndrome. J Am Coll Cardiol 61(2):S. 164–S. 172
Greenberg B, Butler J, Felker GM, Ponikowski P, Voors AA, Desai AS, Barnard D, Bouchard A, Jaski B, Lyon AR, Pogoda JM, Rudy JJ, Zsebo KM (2016) Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2)a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet (London, England) 387(10024):S. 1178–S. 1186
Grunnet M, Bentzen BH, Sorensen US, Diness JG (2012) Cardiac ion channels and mechanisms for protection against atrial fibrillation. Rev Physiol Biochem Pharmacol 162:S. 1–S.58
Hancox JC, James AF, Marrion NV, Zhang H, Thomas D (2016) Novel ion channel targets in atrial fibrillation. Expert Opin Ther Targets 20(8):S. 947–S. 958
Harrell DT, Ashihara T, Ishikawa T, Tominaga I, Mazzanti A, Takahashi K, Oginosawa Y, Abe H, Maemura K, Sumitomo N, Uno K, Takano M, Priori SG, Makita N (2015) Genotype-dependent differences in age of manifestation and arrhythmia complications in short QT syndrome. Int J Cardiol 190:S. 393–S. 402
Hasenfuss G, Meyer M, Schillinger W, Preuss M, Pieske B, Just H (1997) Calcium handling proteins in the failing human heart. Basic Res Cardiol 92(S1):S. 87–S. 93
He J (2001) Reduction in density of transverse tubules and L-type Ca2+ channels in canine tachycardia-induced heart failure. Cardiovasc Res 49(2):S. 298–S. 307
Heijman J, Voigt N, Nattel S, Dobrev D (2014) Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res 114(9):S. 1483–S. 1499
Hoekstra M, Mummery CL, Wilde AA, Bezzina CR, Verkerk AO (2012) Induced pluripotent stem cell derived cardiomyocytes as models for cardiac arrhythmias. Front Physiol 3:346 eCollection 2012
Hong T, Yang H, Zhang SS, Cho HC, Kalashnikova M, Sun B, Zhang H, Bhargava A, Grabe M, Olgin J, Gorelik J, Marbán E, Jan LY, Shaw RM (2014) Cardiac BIN1 folds T-tubule membrane, controlling ion flux and limiting arrhythmia. Nat Med. Jun;20(6):624-32
Hulot JS, Ishikawa K, Hajjar RJ (2016) Gene therapy for the treatment of heart failure promise postponed. Eur Heart J 37(21):S. 1651–S. 1658
Janse M (2004) Electrophysiological changes in heart failure and their relationship to arrhythmogenesis. Cardiovasc Res 61(2):S. 208–S. 217
Kääb S, Nuss HB, Chiamvimonvat N, O'Rourke B, Pak PH, Kass DA, Marban E, Tomaselli GF (1996) Ionic mechanism of action potential prolongation in ventricular myocytes from dogs with pacing-induced heart failure. Circ Res 78(2):S. 262–S. 273
Kaab S, Dixon J, Duc J, Ashen D, Nabauer M, Beuckelmann DJ, Steinbeck G, McKinnon D, Tomaselli GF (1998) Molecular basis of transient outward potassium current downregulation in human heart failure a decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation 98(14):S. 1383–S. 1393
Kattygnarath D, Maugenre S, Neyroud N, Balse E, Ichai C, Denjoy I, Dilanian G, Martins RP, Fressart V, Berthet M, Schott JJ, Leenhardt A, Probst V, Le Marec H, Hainque B, Coulombe A, Hatem SN, Guicheney P (2011) MOG1: a new susceptibility gene for Brugada syndrome. Circ Cardiovasc Genet 4(3):S. 261–S. 268
Kjekshus J (1990) Arrhythmias and mortality in congestive heart failure. Am J Cardiol 65(19):42I–48I
Kostin S (2007) Zonula occludens-1 and connexin 43 expression in the failing human heart. J Cell Mol Med 11(4):S. 892–S. 895
Li D, Melnyk P, Feng J, Wang Z, Petrecca K, Shrier A, Nattel S (2000) Effects of experimental heart failure on atrial cellular and ionic electrophysiology. Circulation 101(22):S. 2631–S. 2638
Li GR, Lau CP, Ducharme A, Tardif JC, Nattel S (2002) Transmural action potential and ionic current remodeling in ventricles of failing canine hearts. Am J Physiol Heart Circ Physiol 283(3):H1031–H1041
Lugenbiel P, Wenz F, Govorov K, Schweizer PA, Katus HA, Thomas D (2015) Atrial fibrillation complicated by heart failure induces distinct remodeling of calcium cycling proteins. PLoS One 10(3):e0116395
Manfra O, Frisk M, Louch WE (2017) Regulation of cardiomyocyte T-tubular structure: opportunities for therapy. Curr Heart Fail Rep 14(3):S. 167–S. 178
Mene-Afejuku TO, López PD, Akinlonu A, Dumancas C, Visco F, Mushiyev S, Pekler G (2018) Atrial Fibrillation in Patients with Heart Failure: Current State and Future Directions. Am J Cardiovasc Drugs. https://doi.org/10.1007/s40256-018-0276-1
Milano A, Vermeer AM, Lodder EM, Barc J, Verkerk AO, Postma AV, van der Bilt IA, Baars MJ, van Haelst PL, Caliskan K, Hoedemaekers YM, Le Scouarnec S, Redon R, Pinto YM, Christiaans I, Wilde AA, Bezzina CR (2014) HCN4 mutations in multiple families with bradycardia and left ventricular noncompaction cardiomyopathy. J Am Coll Cardiol 64(8):S. 745–S. 756
Mohamed U, Napolitano C, Priori SG (2007) Molecular and electrophysiological bases of catecholaminergic polymorphic ventricular tachycardia. J Cardiovasc Electrophysiol 18(7):S. 791–S. 797
Moss AJ, Kass RS (2005) Long QT syndrome from channels to cardiac arrhythmias. J Clin Invest 115(8):S. 2018–S. 2024
Mukherjee R, Hewett KW, Walker JD, Basler CG, Spinale FG (1998) Changes in L-type calcium channel abundance and function during the transition to pacing-induced congestive heart failure. Cardiovasc Res 37(2):S. 432–S. 444
Napolitano C, Priori SG (2007) Diagnosis and treatment of catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 4(5):S. 675–S. 678
Nattel S, Maguy A, Le Bouter S, Yeh YH (2007) Arrhythmogenic ion-channel remodeling in the heart heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev 87(2):S. 425–S. 456
Nattel S, Burstein B, Dobrev D (2008) Atrial remodeling and atrial fibrillation mechanisms and implications. Circ Arrhythm Electrophysiol 1(1):S. 62–S. 73
Nerbonne JM, Kass RS (2005) Molecular physiology of cardiac repolarization. Physiol Rev 85(4):S. 1205–S. 1253
Nuss HB, Kääb S, Kass DA, Tomaselli GF, Marbán E (1999) Cellular basis of ventricular arrhythmias and abnormal automaticity in heart failure. Am J Phys 277(1 Pt 2):H80–H91
Opthof T, Coronel R, Rademaker HM, Vermeulen JT, Wilms-Schopman FJ, Janse MJ (2000) Changes in sinus node function in a rabbit model of heart failure with ventricular arrhythmias and sudden death. Circulation 101(25):S. 2975–S. 2980
Ouadid H, Albat B, Nargeot J (1995) Calcium currents in diseased human cardiac cells. J Cardiovasc Pharmacol 25(2):S. 282–S. 291
Poelzing S, Rosenbaum DS (2004) Altered connexin43 expression produces arrhythmia substrate in heart failure. Am J Physiol Heart Circ Physiol. 287(4):H1762–70
Pogwizd SM, Bers DM (2002) Calcium cycling in heart failure. The arrhythmia connection. J Cardiovasc Electrophysiol 13(1):S. 88–S. 91
Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM (2001) Arrhythmogenesis and contractile dysfunction in heart failure roles of sodium-calcium exchange, inward rectifier potassium current, and residual -adrenergic responsiveness. Circ Res 88(11):S. 1159–S. 1167
Priori SG, Pandit SV, Rivolta I, Berenfeld O, Ronchetti E, Dhamoon A, Napolitano C, Anumonwo J, di Barletta MR, Gudapakkam S, Bosi G, Stramba-Badiale M, Jalife J (2005) A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ Res 96: 800–807
Reynolds JO, Quick AP, Wang Q, Beavers DL, Philippen LE, Showell J, Barreto-Torres G, Thuerauf DJ, Doroudgar S, Glembotski CC, Wehrens XH (2016) Junctophilin-2 gene therapy rescues heart failure by normalizing RyR2-mediated Ca2+ release. Int J Cardiol 225:S. 371–S. 380
Ritterhoff J, Völkers M, Seitz A, Spaich K, Gao E, Peppel K, Pleger ST, Zimmermann WH, Friedrich O, Fink RH, Koch WJ, Katus HA, Most P (2015) S100A1 DNA-based inotropic therapy protects against proarrhythmogenic ryanodine receptor 2 dysfunction. Mol Ther 23(8):S. 1320–S. 1330
Rose J, Armoundas AA, Tian Y, DiSilvestre D, Burysek M, Halperin V, O'Rourke B, Kass DA, Marbán E, Tomaselli GF (2005) Molecular correlates of altered expression of potassium currents in failing rabbit myocardium. Am J Physiol Heart Circ Physiol 288(5):H2077–H2087
Rozanski GJ, Xu Z, Whitney RT, Murakami H, Zucker IH (1997) Electrophysiology of rabbit ventricular myocytes following sustained rapid ventricular pacing. J Mol Cell Cardiol 29(2):S. 721–S. 732
Sanders P, Kistler PM, Morton JB, Spence SJ, Kalman JM (2004) Remodeling of sinus node function in patients with congestive heart failure. Reduction in sinus node reserve. Circulation 110(8):S. 897–S. 903
Schmidt C, Wiedmann F, Kallenberger SM, Ratte A, Schulte JS, Scholz B, Müller FU, Voigt N, Zafeiriou MP, Ehrlich JR, Tochtermann U, Veres G, Ruhparwar A, Karck M, Katus HA, Thomas D (2017) Stretch-activated two-pore-domain (K2P) potassium channels in the heart focus on atrial fibrillation and heart failure. Prog Biophys Mol Biol 130(Pt B):S. 233–S. 243
Schotten U, Verheule S, Kirchhof P, Goette A (2011) Pathophysiological mechanisms of atrial fibrillation a translational appraisal. Physiol Rev 91(1):S. 265–S. 325
Schram G (2002) Differential distribution of cardiac ion channel expression as a basis for regional specialization in electrical function. Circ Res 90(9):S. 939–S. 950
Schwartz PJ, Crotti L, Insolia R (2012) Long-QT syndrome from genetics to management. Circ Arrhythm Electrophysiol 5(4):S. 868–S. 877
Schweizer PA, Schröter J, Greiner S, Haas J, Yampolsky P, Mereles D, Buss SJ, Seyler C, Bruehl C, Draguhn A, Koenen M, Meder B, Katus HA, Thomas D (2014) The symptom complex of familial sinus node dysfunction and myocardial noncompaction is associated with mutations in the HCN4 channel. J Am Coll Cardiol 64(8):S. 757–S. 767
Severs NJ, Bruce AF, Dupont E, Rothery S (2008) Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res 80(1):S. 9–S.19
Tsuji Y (2000) Pacing-induced heart failure causes a reduction of delayed rectifier potassium currents along with decreases in calcium and transient outward currents in rabbit ventricle. Cardiovasc Res 48(2):S. 300–S. 309
Tsuji Y, Zicha S, Qi X-Y, Kodama I, Nattel S (2006) Potassium channel subunit remodeling in rabbits exposed to long-term bradycardia or tachycardia discrete arrhythmogenic consequences related to differential delayed-rectifier changes. Circulation 113(3):S. 345–S. 355
Ufret-Vincenty CA, Baro DJ, Lederer WJ, Rockman HA, Quinones LE, Santana LF (2001) Role of sodium channel deglycosylation in the genesis of cardiac arrhythmias in heart failure. J Biol Chem 276(30):S. 28197–S. 28203
van Rijen HV, Eckardt D, Degen J, Theis M, Ott T, Willecke K, Jongsma HJ, Opthof T, Bakker JM de (2004) Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation 109 (8), S. 1048–S. 1055
Vermeer AMC, Lodder EM, Thomas D, Duijkers FAM, Marcelis C, van Gorselen EOF, Fortner P, Buss SJ, Mereles D, Katus HA, Wilde AAM, Bezzina CR, Boekholdt SM, Schweizer PA, Christiaans I (2016) Dilation of the aorta ascendens forms part of the clinical spectrum of HCN4 mutations. J Am Coll Cardiol 67(19):2313–2315
Vermeulen JT, McGuire MA, Opthof T, Coronel R, de Bakker JM, Klöpping C, Janse MJ (1994) Triggered activity and automaticity in ventricular trabeculae of failing human and rabbit hearts. Cardiovasc Res 28(10):S. 1547–S. 1554
Workman AJ, Kane KA, Rankin AC (2008) Cellular bases for human atrial fibrillation. Heart Rhythm 5(6 Suppl):S. 1–S. 6
Yu S, Li G, Huang CLH, Lei M, Wu L (2018) Late sodium current associated cardiac electrophysiological and mechanical dysfunction. Pflugers Arch - Eur J Physiol 470(3):S. 461–S. 469
Zicha S, Xiao L, Stafford S, Cha TJ, Han W, Varro A, Nattel S (2004) Transmural expression of transient outward potassium current subunits in normal and failing canine and human hearts. J Physiol 561(Pt 3):S. 735–S. 748
Zicha S, Fernández-Velasco M, Lonardo G, L'Heureux N, Nattel S (2005) Sinus node dysfunction and hyperpolarization-activated (HCN) channel subunit remodeling in a canine heart failure model. Cardiovasc Res 66(3):S. 472–S. 481
Funding
This work was supported in part by grants from the University of Heidelberg, Faculty of Medicine (Physician Scientist Scholarship to A.K.R.), from the German Cardiac Society (DGK Scholarship to A.K.R) from the German Cardiac Society and the Hengstberger Foundation (Klaus-Georg and Sigrid Hengstberger Scholarship to D.T.), from the German Heart Foundation/German Foundation of Heart Research (F/08/14 to D.T.), from the Joachim Siebenreicher Foundation (to D.T.), and from the Ministry of Science, Research and the Arts Baden-Wuerttemberg (Sonderlinie Medizin to D.T.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
D.T. reports receiving lecture fees/honoraria from Bayer Vital, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Medtronic, Pfizer Pharma, Sanofi-Aventis, St. Jude Medical, and ZOLL CMS, and research grant support from Daiichi Sankyo.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
This article is part of a Special Issue on “Heart Failure Due to Non-Myofibrillar Defects” edited by Elisabeth Ehler and Katja Gehmlich.
Rights and permissions
About this article
Cite this article
Rahm, AK., Lugenbiel, P., Schweizer, P.A. et al. Role of ion channels in heart failure and channelopathies. Biophys Rev 10, 1097–1106 (2018). https://doi.org/10.1007/s12551-018-0442-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-018-0442-3