Abstract
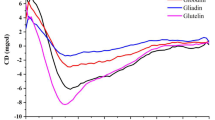
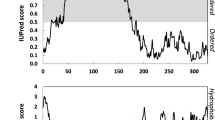
Gliadins are well-known wheat grain proteins, particularly important in food science. They were studied as early as the 1700s. Despite their long history, it has been difficult to identify their higher-order structure as they aggregate in aqueous solution. Consequently, most studies have been performed by extracting the proteins in 70% ethanol or dilute acidic solutions. The carboxy-terminal half of α- and γ-gliadins have α-helix-rich secondary structures stabilized with intramolecular disulfide bonds, which are present in either aqueous ethanol or pure water. The amino-terminal-repeat region of α- and γ-gliadins has poly-L-proline II and β-reverse-turn structures. ω-Gliadins also have poly-L-proline II and β-reverse-turn structures, but no α-helix structure. The size and shape of gliadin molecules have been determined by assessing a variety of parameters: their sedimentation velocity in the analytical ultracentrifuge, intrinsic viscosity, small-angle X-ray scattering profile, and images of the proteins from scanning probe microscopes such as a tunneling electron microscope and atomic force microscope. Models for gliadins are either rods or prolate ellipsoids whether in aqueous ethanol, dilute acid, or pure water. Recently, gliadins have been shown to be soluble in pure water, and a novel extraction method into pure water has been established. This has made it possible to analyze gliadins in pure water at neutral pH, and permitted the characterization of hydrated gliadins. They formed hierarchical nanoscale structures with internal density fluctuations at high protein concentrations.




Similar content being viewed by others
References
Altenbach SB, Kothari KM (2007) Omega gliadin genes expressed in Triticum aestivum cv. Butte 86: effects of post-anthesis fertilizer on transcript accumulation during grain development. J Cereal Sci 46:169–177
Altschuler Y, Galili G (1994) Role of conserved cysteines of a wheat gliadin in its transport and assembly into protein bodies in xenopus oocytes. J Biol Chem 269:6677–6682
Anderson OD, Greene FC (1997) The α-gliadin gene family. II. DNA and protein sequence variation, subfamily structure, and origins of pseudogenes. Theor Appl Genet 95:59–65
Anderson OD, Hsia CC, Torres V (2001) The wheat γ-gliadin genes: characterization of ten new sequences and further understanding of γ-gliadin gene family structure. Theor Appl Genet 103:323–330
Ang S, Kogulanathan J, Morris AM, Kök MS, Shewry PR, Tatham AS, Adams GG, Rowe AJ, Harding SE (2010) Structure and heterogeneity of gliadin: a hydrodynamic evaluation. Eur Biophys J 39:255–261
Arêas EPG, Cassiano MM (2001) Folding interpenetration in a gliadin model: the role of the characteristic octapeptide motif. Biophys Chem 90:135–146
Bailey CH (1941) A translation of Beccari's lecture “concerning grain” (1728). J Cereal Chem 18:555–561
Blanch EW, Kasarda DD, Hecht L, Nielsen K, Barron LD (2003) New insight into the solution structures of wheat gluten proteins from Raman optical activity. Biochemistry 42:5665–5673
Bulleid NJ, Freedman RB (1988) Defective co-translational formation of disulphide bonds in protein disulphide-isomerase-deficient microsomes. Nature 335:649–651
Chittenden RH, Folin O, Gies WJ, Koch W, Osborne TB, Osborne TB, Levene PA, Mandel JA, Mathews AP, Mendel LB (1908) Joint recommendations of the physiological and biochemical committees on protein nomenclature. Science 27:554–556
Clements RL (1973) Effects of prior salt treatment on gluten dispersibility. Cereal Chem 50:87–100
Cole EW, Kasarda DD, Lafiandra D (1984) The conformational structure of a-gliadin intrinsic viscosities under conditions approaching the native state and under denaturing conditions. Biochim Biophys Acta 787:244–251
Fu BX, Sapirstein HD, Bushuk W (1996) Salt-induced disaggregation / solubilization of gliadin and glutenin proteins in water. J Cereal Sci 24:241–246
Hirota N, Mizuno K, Goto U (1999) Group additive contributions to the alcohol-induced α-helix formation of melittin: implication for the mechanism of the alcohol effects on proteins. J Mol Biol 275:365–378
Hsia CC, Anderson OD (2001) Isolation and characterization of wheat -gliadin genes. Theor Appl Genet 103:37–44
I'Anson KJ, Morris VJ, Shewry PR, Tatham AS (1992) Small-angle X-ray-scattering studies of the C hordeins of barley (Hordeum vulgare). Biochem J 287:183–185
Imai T, Kovalemko A, Hirata F, Kidera A (2009) Molecular thermodynamics of trifluoroethanol-induced helix formation: analysis of the solvation structure and free energy by the 3D-RISM theory. Interdiscip Sci 1:156–160
Kasarda DD (1980) Structure and properties of α-gliadin. Annal Technol Agric 29:151–173
Kasarda DD, Bernardin JE, Thomas RS (1967) Reversible aggregation of α-gliadin to fibrils. Science 155:203–205
Kasarda DD, Bernardin JE, Gaffield W (1968) Circular dichroism and optical rotatory dispersion of α-gliadin. Biochemistry 7:3950–3957
Kasarda DD, Adalstein AE, Laird NF (1987) γ-Gliadins with α-type structure coded on chromosome 6B of the wheat (Triticum aestivum L.) cultivar ‘Chinese spring’. In: Lásztity R, Békés F (eds) Proc. 3rd Int. workshop on gluten proteins. World Scientific Publishing, Singapore, pp 20–29
Kimura S, Higashino Y, Kitao Y, Masuda T, Urade R (2015) Expression and characterization of protein disulfide isomerase family proteins in bread wheat. BMC Plant Biol 15:73
Kohn JE, Millett IS, Jacob J, Zagrovic B, Dillon TM, Cingel N, Dothager RS, Seifert S, Thiyagarajan P, Sosnick TR, Hasan MZ, Pande VS, Ruczinski I, Doniach S, Plaxco KW (2004) Random-coil behavior and the dimensions of chemically unfolded proteins. Proc Natl Acad Sci U S A 101:12491–12496
Lawrence G, MacRitchie F, Wrigley CW (1988) Dough and baking quality of wheat lines deficient in glutenin subunits controlled by the Glu-Al, Glu-Bland Glu-Dl loci. J Cereal Sci 7:109–112
León A, Rosell CM, Barber CB (2003) A differential scanning calorimetry study of wheat proteins. Eur Food Res Technol 217:13–16
Lindsay MP, Skerritt JH (2000) Immunocytochemical localization of gluten proteins uncovers structural organization of glutenin macropolymer. Cereal Chem 77:360–369
Matsuo H, Kohno K, Morita E (2005) Molecular cloning, recombinant expression and IgE binding ω-5 gliadin is a major allergen in wheat-dependent exercise-induced anaphylaxis. FEBS J 272:4431–4438
Matsushima N, Creutz CE, Kretsinger RH (1990) Polyproline, β-turn helices. Novel secondary structures proposed for the tandem repeat within rhodopsin, synaptophysin, synexin, gliadin, RNA polymerase II, hordein, and glute. Proteins Struct Funct Genet 7:125–155
McMaster TJ, Miles MJ, Kasarda DD, Shewry PR, Tatham AS (1999a) Atomic force microscopy of A-gliadin fibrils and in situ degradation. J Cereal Sci 31:281–286
McMaster TJ, Miles MJ, Wannerberger L, Eliasson A-C, Shewry PR, Tatham AS (1999b) Identification of microphases in mixed α- and ω-gliadin protein films investigated by atomic force microscopy. J Agric Food Chem 47:5093–5099
Müller S, Wieser H (1995) The location of disulphide bonds in γ-type gliadins. J Cereal Sci 22:21–27
Müller S, Wieser H (1997) The location of disulfide bonds in monomeric γ-type gliadins. J Cereal Sci 26:169–176
Orsi A, Sparvoli F, Ceriotti A (2001) Role of individual disulfide bonds in the structural maturation of a low molecular weight glutenin subunit. J Biol Chem 276:32322–32329
Osborne TB (1924) The vegetable proteins, 2nd edn. Longmans Green, London
Payne P (1987) Genetics of wheat storage proteins and the effect of allelic variation on bread-making quality. Ann Rev Plant Physiol 38:141–153
Sabelli P, Shewry PR (1991) Characterization and organization of gene families at the Gli-1 loci of bread and durum wheats by restriction fragment analysis. Theor Appl Genet 83:209–216
Sato N, Matsumiya A, Higashino Y, Funaki S, Kitao Y, Oba Y, Inoue R, Arisaka F, Sugiyama M, Urade R (2015) Molecular assembly of wheat gliadins into nanostructures: a small-angle x-ray scattering study of gliadins in distilled water over a wide concentration range. J Agric Food Chem 63:8715–8721
Shewry PR, Tatham AS (1997) Disulphide bonds in wheat gluten proteins. J Cereal Sci 25:207–227
Shewry PR, Miflin BJ, Kasarda DD (1984) The structural and evolutionary relationships of the prolamin storage proteins of barley, rye and wheat. Philos Trans R Soc Lond B 304:297–308
Shewry PR, Tatham AS, Forde J, Kreis M, Miflin BJ (1986) The classification and nomenclature of wheat gluten proteins: a reassessment. J Cereal Sci 4:97–106
Shewry PR, Miles MJ, Tompson NH, Tatham AS (1997) Scanning probe microscopes - applications in cereal science. Cereal Chem 74:193–199
Shewry PR, D’Ovidio R, Lafiandra D, Jenkins JA, Mills ENC, Békés F (2009) Wheat grain proteins. In: Khan K, Shewry PR (eds) Wheat: chemistry and technology, 4th edn. AACC International, Minnesota, pp 223–298
Shimoni Y, Galili G (1996) Intramolecular disulfide bonds between conserved cysteines in wheat gliadins control their deposition into protein bodies. J Biol Chem 271:18869–18874
Taddei G (1819) Ricerchesulglutine del frumento. Giornale di fisica, chimica, e storianaturale. Brugnatelli 2:360–361
Tatham AS, Shewry PR (1995) The S-poor prolamins. J Cereal Sci 2:99–103
Tatham AS, Drake AF, Shewry PR (1985a) A conformational study of a glutamine-rich and proline-rich cereal seed protein, C-hordein. Biochem J 226:557–562
Tatham AS, Miflin B, Shewry PR (1985b) The β-turn conformation in wheat gluten proteins - relationship to gluten elasticity. Cereal Chem 62:405–411
Tatham AS, Field JM, Smith SJ, Shewry PR (1987) The conformations of wheat gluten proteins, II*, aggregated gliadins and low molecular weight subunits of glutenin. J Cereal Sci 5:203–214
Tatham AS, Tomson NH, McMaster TJ, Humphris ADL, Miles MJ (1999) Scanning probe microscopy studies of cereal seed storage protein structures. Scanning 21:293–298
Thomson NH, Miles MJ, Tatham AS, Shewry PR (1992) Molecular images of cereal proteins by STM. Ultramicroscopy 42-44(Pt B):1204–1213
Thomson NH, Miles MJ, Popineau Y, Harries J, Shewry PR, Tatham AS (1999) Small angle X-ray scattering of wheat seed-storage proteins: α-, γ- and ω-gliadins and the high molecular weight (HMW) subunits of glutenin. Biochim Biophys Acta 1430:359–366
Ukai T, Matsumura Y, Urade R (2008) Disaggregation and reaggregation of gluten proteins by sodium chloride. J Agric Food Chem 56:1122–1130
Uthayakumaran S, Newberry M, Keentok M, Stoddard FL, Bekes F (2000) Basic rheology of bread dough with modified protein content and glutenin-to-gliadin ratios. Cereal Chem 77:744–749
Wrigley CW, Shepherd KW (1973) Electrofocusing of grain proteins from wheat genotypes. Ann N Y Acad Sci 209:154–162
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 26660111 to R.U, JP15H02042 to M.S. and a grant from the Tojuro Iijima Foundation for Food Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Reiko Urade declares that she has no conflicts of interest. Nobuhiro Sato declares that he has no conflicts of interest. Masaaki Sugiyama declares that he has no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
This article is part of a Special Issue on ‘Biomolecules to Bio-nanomachines - Fumio Arisaka 70th Birthday’ edited by Damien Hall, Junichi Takagi and Haruki Nakamura.
Rights and permissions
About this article
Cite this article
Urade, R., Sato, N. & Sugiyama, M. Gliadins from wheat grain: an overview, from primary structure to nanostructures of aggregates. Biophys Rev 10, 435–443 (2018). https://doi.org/10.1007/s12551-017-0367-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-017-0367-2