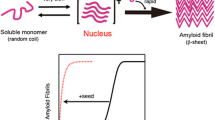
Abstract
Amyloid fibrils are supramolecular protein assemblies with a fibrous morphology and cross-β structure. The formation of amyloid fibrils typically follows a nucleation-dependent polymerization mechanism, in which a one-step nucleation scheme has widely been accepted. However, a variety of oligomers have been identified in early stages of fibrillation, and a nucleated conformational conversion (NCC) mechanism, in which oligomers serve as a precursor of amyloid nucleation and convert to amyloid nuclei, has been proposed. This development has raised the need to consider more complicated multi-step nucleation processes in addition to the simplest one-step process, and evidence for the direct involvement of oligomers as nucleation precursors has been obtained both experimentally and theoretically. Interestingly, the NCC mechanism has some analogy with the two-step nucleation mechanism proposed for inorganic and organic crystals and protein crystals, although a more dramatic conformational conversion of proteins should be considered in amyloid nucleation. Clarifying the properties of the nucleation precursors of amyloid fibrils in detail, in comparison with those of crystals, will allow a better understanding of the nucleation of amyloid fibrils and pave the way to develop techniques to regulate it.





Similar content being viewed by others
References
Arosio P, Knowles TP, Linse S (2015) On the lag phase in amyloid fibril formation. Phys Chem Chem Phys 17:7606–7618
Auer S, Dobson CM, Vendruscolo M (2007) Characterization of the nucleation barriers for protein aggregation and amyloid formation. HFSP J 1:137–146
Auer S, Ricchiuto P, Kashchiev D (2012) Two-step nucleation of amyloid fibrils: omnipresent or not? J Mol Biol 422:723–730
Baftizadeh F, Biarnes X, Pietrucci F, Affinito F, Laio A (2012) Multidimensional view of amyloid fibril nucleation in atomistic detail. J Am Chem Soc 134:3886–3894
Bitan G, Teplow DB (2004) Rapid photochemical cross-linking--a new tool for studies of metastable, amyloidogenic protein assemblies. Acc Chem Res 37:357–364
Bleiholder C, Dupuis NF, Wyttenbach T, Bowers MT (2011) Ion mobility-mass spectrometry reveals a conformational conversion from random assembly to β-sheet in amyloid fibril formation. Nat Chem 3:172–177
Breydo L, Uversky VN (2015) Structural, morphological, and functional diversity of amyloid oligomers. FEBS Lett 589:2640–2648
Cabriolu R, Kashchiev D, Auer S (2010) Atomistic theory of amyloid fibril nucleation. J Chem Phys 133:225101
Chatani E, Goto Y (2005) Structural stability of amyloid fibrils of β2-microglobulin in comparison with its native fold. Biochim Biophys Acta 1753:64–75
Chatani E, Imamura H, Yamamoto N, Kato M (2014) Stepwise organization of the β-structure identifies key regions essential for the propagation and cytotoxicity of insulin amyloid fibrils. J Biol Chem 289:10399–10410
Chatani E, Inoue R, Imamura H, Sugiyama M, Kato M, Yamamoto M, Nishida K, Kanaya T (2015) Early aggregation preceding the nucleation of insulin amyloid fibrils as monitored by small angle X-ray scattering. Sci Rep 5:15485
Chattopadhyay S, Erdemir D, Evans JMB, Ilavsky J, Amenitsch H, Segre CU, Myerson AS (2005) SAXS study of the nucleation of glycine crystals from a supersaturated solution. Cryst Growth Des 5:523–527
Chimon S, Shaibat MA, Jones CR, Calero DC, Aizezi B, Ishii Y (2007) Evidence of fibril-like β-sheet structures in a neurotoxic amyloid intermediate of Alzheimer's β-amyloid. Nat Struct Mol Biol 14:1157–1164
Cohen SI, Vendruscolo M, Dobson CM, Knowles TP (2012) From macroscopic measurements to microscopic mechanisms of protein aggregation. J Mol Biol 421:160–171
Crespo R, Rocha FA, Damas AM, Martins PM (2012) A generic crystallization-like model that describes the kinetics of amyloid fibril formation. J Biol Chem 287:30585–30594
Dobson CM (2003) Protein folding and misfolding. Nature 426:884–890
Eghiaian F, Daubenfeld T, Quenet Y, van Audenhaege M, Bouin AP, van der Rest G, Grosclaude J, Rezaei H (2007) Diversity in prion protein oligomerization pathways results from domain expansion as revealed by hydrogen/deuterium exchange and disulfide linkage. Proc Natl Acad Sci USA104:7414–7419
Erdemir D, Lee AY, Myerson AS (2009) Nucleation of crystals from solution: classical and two-step models. Acc Chem Res 42:621–629
Ferrone F (1999) Analysis of protein aggregation kinetics. Methods Enzymol 309:256–274
Fowler DM, Koulov AV, Balch WE, Kelly JW (2007) Functional amyloid—from bacteria to humans. Trends Biochem Sci 32:217–224
Garcia GA, Cohen SI, Dobson CM, Knowles TP (2014) Nucleation-conversion-polymerization reactions of biological macromolecules with prenucleation clusters. Phys Rev E Stat Nonlinear Soft Matter Phys 89:032712
Gebauer D, Kellermeier M, Gale JD, Bergstrom L, Colfen H (2014) Pre-nucleation clusters as solute precursors in crystallisation. Chem Soc Rev 43:2348–2371
Groenning M, Frokjaer S, Vestergaard B (2009) Formation mechanism of insulin fibrils and structural aspects of the insulin fibrillation process. Curr Protein Pept Sci 10:509–528
Harano K, Homma T, Niimi Y, Koshino M, Suenaga K, Leibler L, Nakamura E (2012) Heterogeneous nucleation of organic crystals mediated by single-molecule templates. Nat Mater 11:877–881
Hofrichter J, Ross PD, Eaton WA (1974) Kinetics and mechanism of deoxyhemoglobin S gelation: a new approach to understanding sickle cell disease. Proc Natl Acad Sci USA 71:4864–4868
Hsieh MC, Lynn DG, Grover MA (2017) Kinetic model for two-step nucleation of peptide assembly. J Phys Chem B 121:7401–7411
Jarrett JT, Lansbury PT Jr (1993) Seeding "one-dimensional crystallization" of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell 73:1055–1058
Johnson SM, Connelly S, Fearns C, Powers ET, Kelly JW (2012) The transthyretin amyloidoses: from delineating the molecular mechanism of aggregation linked to pathology to a regulatory-agency-approved drug. J Mol Biol 421:185–203
Kamihira M, Naito A, Tuzi S, Nosaka AY, Saito H (2000) Conformational transitions and fibrillation mechanism of human calcitonin as studied by high-resolution solid-state 13C NMR. Protein Sci 9:867–877
Kashchiev D, Auer S (2010) Nucleation of amyloid fibrils. J Chem Phys 132:215101
Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300:486–489
Kelly JW (2000) Mechanisms of amyloidogenesis. Nat Struct Biol 7:824–826
Laganowsky A, Liu C, Sawaya MR, Whitelegge JP, Park J, Zhao M, Pensalfini A, Soriaga AB, Landau M, Teng PK, Cascio D, Glabe C, Eisenberg D (2012) Atomic view of a toxic amyloid small oligomer. Science 335:1228–1231
Lee J, Culyba EK, Powers ET, Kelly JW (2011) Amyloid-β forms fibrils by nucleated conformational conversion of oligomers. Nat Chem Biol 7:602–609
Loh ND, Sen S, Bosman M, Tan SF, Zhong J, Nijhuis CA, Kral P, Matsudaira P, Mirsaidov U (2017) Multistep nucleation of nanocrystals in aqueous solution. Nat Chem 9:77–82
Lomakin A, Chung DS, Benedek GB, Kirschner DA, Teplow DB (1996) On the nucleation and growth of amyloid β-protein fibrils: detection of nuclei and quantitation of rate constants. Proc Natl Acad Sci USA 93:1125–1129
Maes D, Vorontsova MA, Potenza MA, Sanvito T, Sleutel M, Giglio M, Vekilov PG (2015) Do protein crystals nucleate within dense liquid clusters? Acta Crystallogr F Struct Biol Commun 71:815–822
Maury CP (2009) The emerging concept of functional amyloid. J Intern Med 265:329–334
Meisl G, Kirkegaard JB, Arosio P, Michaels TC, Vendruscolo M, Dobson CM, Linse S, Knowles TP (2016) Molecular mechanisms of protein aggregation from global fitting of kinetic models. Nat Protoc 11:252–272
Morris AM, Watzky MA, Finke RG (2009) Protein aggregation kinetics, mechanism, and curve-fitting: a review of the literature. Biochim Biophys Acta 1794:375–397
Nors Pedersen M, Fodera V, Horvath I, van Maarschalkerweerd A, Norgaard Toft K, Weise C, Almqvist F, Wolf-Watz M, Wittung-Stafshede P, Vestergaard B (2015) Direct correlation between ligand-induced α-synuclein oligomers and amyloid-like fibril growth. Sci Rep 5:10422
Oosawa F, Kasai M (1962) A theory of linear and helical aggregations of macromolecules. J Mol Biol 4:10–21
Paslawski W, Mysling S, Thomsen K, Jorgensen TJ, Otzen DE (2014) Co-existence of two different α-synuclein oligomers with different core structures determined by hydrogen/deuterium exchange mass spectrometry. Angew Chem Int Ed Engl 53:7560–7563
Pavlova A, Cheng CY, Kinnebrew M, Lew J, Dahlquist FW, Han S (2016) Protein structural and surface water rearrangement constitute major events in the earliest aggregation stages of tau. Proc Natl Acad Sci USA 113:E127–E136
Potapov A, Yau WM, Ghirlando R, Thurber KR, Tycko R (2015) Successive stages of amyloid-β self-assembly characterized by solid-state nuclear magnetic resonance with dynamic nuclear polarization. J Am Chem Soc 137:8294–8307
Riek R, Eisenberg DS (2016) The activities of amyloids from a structural perspective. Nature 539:227–235
Saric A, Chebaro YC, Knowles TP, Frenkel D (2014) Crucial role of nonspecific interactions in amyloid nucleation. Proc Natl Acad Sci USA 111:17869–17874
Saric A, Michaels TCT, Zaccone A, Knowles TPJ, Frenkel D (2016) Kinetics of spontaneous filament nucleation via oligomers: insights from theory and simulation. J Chem Phys 145:211926
Sauter A, Roosen-Runge F, Zhang F, Lotze G, Jacobs RM, Schreiber F (2015) Real-time observation of nonclassical protein crystallization kinetics. J Am Chem Soc 137:1485–1491
Serio TR, Cashikar AG, Kowal AS, Sawicki GJ, Moslehi JJ, Serpell L, Arnsdorf MF, Lindquist SL (2000) Nucleated conformational conversion and the replication of conformational information by a prion determinant. Science 289:1317–1321
Sipe JD, Benson MD, Buxbaum JN, Ikeda S, Merlini G, Saraiva MJ, Westermark P (2014) Nomenclature 2014: amyloid fibril proteins and clinical classification of the amyloidosis. Int J Exp Clin Invest 21:221–224
So M, Hall D, Goto Y (2016) Revisiting supersaturation as a factor determining amyloid fibrillation. Curr Opin Struct Biol 36:32–39
Sosso GC, Chen J, Cox SJ, Fitzner M, Pedevilla P, Zen A, Michaelides A (2016) Crystal nucleation in liquids: open questions and future challenges in molecular dynamics simulations. Chem Rev 116:7078–7116
Tycko R (2014) Physical and structural basis for polymorphism in amyloid fibrils. Protein Sci 23:1528–1539
Vekilov PG (2010) The two-step mechanism of nucleation of crystals in solution. Nano 2:2346–2357
Vekilov PG, Vorontsova MA (2014) Nucleation precursors in protein crystallization. Acta Crystallogr F Struct Biol Commun 70:271–282
Vestergaard B, Groenning M, Roessle M, Kastrup JS, van de Weert M, Flink JM, Frokjaer S, Gajhede M, Svergun DI (2007) A helical structural nucleus is the primary elongating unit of insulin amyloid fibrils. PLoS Biol 5:e134
Vivares D, Kaler EW, Lenhoff AM (2005) Quantitative imaging by confocal scanning fluorescence microscopy of protein crystallization via liquid-liquid phase separation. Acta Crystallogr D Biol Crystallogr 61:819–825
Yoshimura Y, Lin Y, Yagi H, Lee YH, Kitayama H, Sakurai K, So M, Ogi H, Naiki H, Goto Y (2012) Distinguishing crystal-like amyloid fibrils and glass-like amorphous aggregates from their kinetics of formation. Proc Natl Acad Sci USA 109:14446–14451
Young LM, Ashcroft AE, Radford SE (2017) Small molecule probes of protein aggregation. Curr Opin Chem Biol 39:90–99
Zhang TH, Liu XY (2007) How does a transient amorphous precursor template crystallization. J Am Chem Soc 129:13520–13526
Acknowledgements
We wish to express our congratulations to Professor Fumio Arisaka on his 70th birthday, and we thank the editors for the great opportunity to participate in this special issue. We thank Dr. Hiroshi Imamura (Ritsumeikan Universiy) for his helpful comments and discussions on the earlier draft of this article. This work was supported by JSPS KAKENHI Grant Numbers JP16H04778, JP16H00772, JP16K17783, and JP17H06352.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Eri Chatani declares that she has no conflicts of interest. Naoki Yamamoto declares that he has no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
This article is part of a Special Issue on ‘Biomolecules to Bio-nanomachines—Fumio Arisaka 70th Birthday’ edited by Damien Hall, Junichi Takagi and Haruki Nakamura.
Rights and permissions
About this article
Cite this article
Chatani, E., Yamamoto, N. Recent progress on understanding the mechanisms of amyloid nucleation. Biophys Rev 10, 527–534 (2018). https://doi.org/10.1007/s12551-017-0353-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-017-0353-8