Abstract
Lung cancers are broadly classified into small cell lung cancers and non-small cell lung cancers (NSCLC). Lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) are two common subtypes of NSCLC, and despite the fact that both occur in lung tissues, these two subtypes show a number of different pathological characteristics. To investigate the differences and seek potential therapy targets, we used bioinformatics methods to analyze RNA-Seq data from different aspects. The previous studies and comparative pathway enrichment analysis on publicly available data showed that expressed or inhibited genes are different in two cancer subtypes through important pathways. Some of these genes could not only affect cell function through expression, but also could regulate other genes’ expression by binding to a specific DNA sequence. This kind of genes is called transcription factor (TF) or sequence-specific DNA-binding factor. Transcription factors play important roles in controlling gene expression in carcinoma pathways. Our results revealed transcription factors that may cause differential expression of genes in cellular pathways of LUAD and LUSC, which provide new clues for study and treatment. Once such TF is NFE2l2 which may regulate genes in the Wnt signaling pathway, and the MAPK signaling pathway, thus leading to an increase the cell growth, cell division, and gene transcription. Another TF-XBP1 has high correlation with genes related to cell adhesion molecules and cytokine–cytokine receptor interaction pathways that may further affect the immune system. Moreover, the two TF and high correlated genes also show similar patterns in an independent GEO data set.





Similar content being viewed by others
Abbreviations
- LUAD:
-
Lung adenocarcinoma
- LUSC:
-
Lung squamous cell carcinoma
- NSCLC:
-
Non-small cell lung carcinoma
- GSEA:
-
Gene set enrichment analysis
- FDR:
-
False discovery rate
References
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA (2008) Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 83(5):584–594
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67(1):7–30
Chang JT, Lee YM, Huang RS (2015) The impact of the Cancer Genome Atlas on lung cancer. Transl Res 166(6):568–585
Subramanian J, Govindan R (2007) Lung cancer in never smokers: a review. J Clin Oncol 25(5):561–570
Hoda SA, Cheng E (2017) Robbins basic pathology. Am J Clin Pathol. https://doi.org/10.1093/ajcp/aqx095
Kawase A, Yoshida J, Ishii G, Nakao M, Aokage K, Hishida T, Nishimura M, Nagai K (2012) Differences between squamous cell carcinoma and adenocarcinoma of the lung: are adenocarcinoma and squamous cell carcinoma prognostically equal? Jpn J Clin Oncol 42(3):189–195
Cancer Genome Atlas Research N (2014) Comprehensive molecular profiling of lung adenocarcinoma. Nature 511(7511):543–550
Cancer Genome Atlas Research N (2012) Comprehensive genomic characterization of squamous cell lung cancers. Nature 489(7417):519–525
Johnston SJ, Carroll JS (2015) Transcription factors and chromatin proteins as therapeutic targets in cancer. Biochimica et biophysica acta 1855(2):183–192
Black AR, Black JD, Azizkhan-Clifford J (2001) Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol 188(2):143–160
Baldwin AS (2001) Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Investig 107(3):241–246
Hsieh YS, Lee YL, Yang SF, Yang JS, Chen W, Chen SC, Shih CM (2007) Association of EcoRI polymorphism of the metastasis-suppressor gene NME1 with susceptibility to and severity of non-small cell lung cancer. Lung Cancer 58(2):191–195
Tokunaga K, Nakamura Y, Sakata K, Fujimori K, Ohkubo M, Sawada K, Sakiyama S (1987) Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Res 47(21):5616–5619
Buhrens RI, Amelung JT, Reymond MA, Beshay M (2009) Protein expression in human non-small cell lung cancer: a systematic database. Pathobiology 76(6):277–285
Dasanu CA, Sethi N, Ahmed N (2012) Immune alterations and emerging immunotherapeutic approaches in lung cancer. Expert Opin Biol Ther 12(7):923–937
Cai B, Jiang X (2014) Revealing biological pathways implicated in lung cancer from TCGA gene expression data using gene set enrichment analysis. Cancer Inform 13(Suppl 1):113–121
Chen M, Liu X, Du J, Wang XJ, Xia L (2017) Differentiated regulation of immune-response related genes between LUAD and LUSC subtypes of lung cancers. Oncotarget 8(1):133–144
Kendall J, Liu Q, Bakleh A, Krasnitz A, Nguyen KC, Lakshmi B, Gerald WL, Powers S, Mu D (2007) Oncogenic cooperation and coamplification of developmental transcription factor genes in lung cancer. Proc Natl Acad Sci U S A 104(42):16663–16668
Halmos B, Huettner CS, Kocher O, Ferenczi K, Karp DD, Tenen DG (2002) Down-regulation and antiproliferative role of C/EBPalpha in lung cancer. Cancer Res 62(2):528–534
Wikman H, Kettunen E (2006) Regulation of the G1/S phase of the cell cycle and alterations in the RB pathway in human lung cancer. Expert Rev Anticancer Ther 6(4):515–530
Kaye FJ (2002) RB and cyclin dependent kinase pathways: defining a distinction between RB and p16 loss in lung cancer. Oncogene 21(45):6908–6914
Ghadersohi A, Odunsi K, Zhang S, Azrak RG, Bundy BN, Manjili MH, Li F (2008) Prostate-derived Ets transcription factor as a favorable prognostic marker in ovarian cancer patients. Int J Cancer 123(6):1376–1384
Kunderfranco P, Mello-Grand M, Cangemi R, Pellini S, Mensah A, Albertini V, Malek A, Chiorino G, Catapano CV, Carbone GM (2010) ETS transcription factors control transcription of EZH2 and epigenetic silencing of the tumor suppressor gene Nkx3.1 in prostate cancer. PloS One 5(5):e10547
Sood AK, Wang J, Mhawech-Fauceglia P, Jana B, Liang P, Geradts J (2009) Sam-pointed domain containing Ets transcription factor in luminal breast cancer pathogenesis. Cancer Epidemiol Biomark Prev 18(6):1899–1903
Dubey R, Saini N (2015) STAT6 silencing up-regulates cholesterol synthesis via miR-197/FOXJ2 axis and induces ER stress-mediated apoptosis in lung cancer cells. Biochimica et biophysica acta 1849(1):32–43
Yang Q, Cao X, Tao G, Zhou F, Zhao P, Shen Y, Chen X (2017) Effects of FOXJ2 on TGF-beta1-induced epithelial–mesenchymal transition through Notch signaling pathway in non-small lung cancer. Cell Biol Int 41(1):79–83
Abazeed ME, Adams DJ, Hurov KE, Tamayo P, Creighton CJ, Sonkin D, Giacomelli AO, Du C, Fries DF, Wong KK et al (2013) Integrative radiogenomic profiling of squamous cell lung cancer. Cancer Res 73(20):6289–6298
Li H, Chen X, Gao Y, Wu J, Zeng F, Song F (2015) XBP1 induces snail expression to promote epithelial- to-mesenchymal transition and invasion of breast cancer cells. Cell Signal 27(1):82–89
Zhang Y, Xuan J, de los Reyes BG, Clarke R, Ressom HW (2008) Network motif-based identification of transcription factor–target gene relationships by integrating multi-source biological data. BMC Bioinformat 9:203
He J, Dai XB, Zhao XC (2006) A systematic computational approach for transcription factor target gene prediction. In: Proceedings of the 2006 IEEE symposium on computational intelligence in bioinformatics and computational biology, pp 385–391
Gordan R, Hartemink AJ, Bulyk ML (2009) Distinguishing direct versus indirect transcription factor–DNA interactions. Genome Res 19(11):2090–2100
Sun F, Yang X, Jin Y, Chen L, Wang L, Shi M, Zhan C, Shi Y, Wang Q (2017) Bioinformatics analyses of the differences between lung adenocarcinoma and squamous cell carcinoma using The Cancer Genome Atlas expression data. Mol Med Rep 16(1):609–616
Rada P, Rojo AI, Offergeld A, Feng GJ, Velasco-Martin JP, Gonzalez-Sancho JM, Valverde AM, Dale T, Regadera J, Cuadrado A (2015) WNT-3A regulates an Axin1/NRF2 complex that regulates antioxidant metabolism in hepatocytes. Antioxid Redox Signal 22(7):555–571
Huang Y, Liu G, Zhang B, Xu G, Xiong W, Yang H (2010) Wnt-5a regulates proliferation in lung cancer cells. Oncol Rep 23(1):177–181
Polakis P (2000) Wnt signaling and cancer. Genes Dev 14(15):1837–1851
Chung SS, Ahn BY, Kim M, Choi HH, Park HS, Kang S, Park SG, Kim YB, Cho YM, Lee HK et al (2010) Control of adipogenesis by the SUMO-specific protease SENP2. Mol Cell Biol 30(9):2135–2146
Nishita M, Hashimoto MK, Ogata S, Laurent MN, Ueno N, Shibuya H, Cho KW (2000) Interaction between Wnt and TGF-beta signalling pathways during formation of Spemann’s organizer. Nature 403(6771):781–785
Zhang S, Guo D, Luo W, Zhang Q, Zhang Y, Li C, Lu Y, Cui Z, Qiu X (2010) TrkB is highly expressed in NSCLC and mediates BDNF-induced the activation of Pyk2 signaling and the invasion of A549 cells. BMC Cancer 10:43
Kamai T, Shirataki H, Nakanishi K, Furuya N, Kambara T, Abe H, Oyama T, Yoshida K (2010) Increased Rac1 activity and Pak1 overexpression are associated with lymphovascular invasion and lymph node metastasis of upper urinary tract cancer. BMC Cancer 10:164
Perl AK, Dahl U, Wilgenbus P, Cremer H, Semb H, Christofori G (1999) Reduced expression of neural cell adhesion molecule induces metastatic dissemination of pancreatic beta tumor cells. Nat Med 5(3):286–291
Rajaraman P, Brenner AV, Neta G, Pfeiffer R, Wang SS, Yeager M, Thomas G, Fine HA, Linet MS, Rothman N et al (2010) Risk of meningioma and common variation in genes related to innate immunity. Cancer Epidemiol Biomark Prev 19(5):1356–1361
Enjuanes A, Benavente Y, Bosch F, Martin-Guerrero I, Colomer D, Perez-Alvarez S, Reina O, Ardanaz MT, Jares P, Garcia-Orad A et al (2008) Genetic variants in apoptosis and immunoregulation-related genes are associated with risk of chronic lymphocytic leukemia. Cancer Res 68(24):10178–10186
Liu CY, Wang YM, Wang CL, Feng PH, Ko HW, Liu YH, Wu YC, Chu Y, Chung FT, Kuo CH et al (2010) Population alterations of l-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14(−)/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol 136(1):35–45
Shibata K, Mori M, Tanaka S, Kitano S, Akiyoshi T (1998) Identification and cloning of human G-protein gamma 7, down-regulated in pancreatic cancer. Biochem Biophys Res Commun 246(1):205–209
Chae YK, Chang S, Ko T, Anker J, Agte S, Iams W, Choi WM, Lee K, Cruz M (2018) Epithelial–mesenchymal transition (EMT) signature is inversely associated with T-cell infiltration in non-small cell lung cancer (NSCLC). Sci Rep 8(1):2918
Homma S, Ishii Y, Morishima Y, Yamadori T, Matsuno Y, Haraguchi N, Kikuchi N, Satoh H, Sakamoto T, Hizawa N et al (2009) Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin Cancer Res 15(10):3423–3432
Chian S, Li YY, Wang XJ, Tang XW (2014) Luteolin sensitizes two oxaliplatin-resistant colorectal cancer cell lines to chemotherapeutic drugs via inhibition of the Nrf2 pathway. Asian Pac J Cancer Prev APJCP 15(6):2911–2916
Ming J, Ruan S, Wang M, Ye D, Fan N, Meng Q, Tian B, Huang T (2015) A novel chemical, STF-083010, reverses tamoxifen-related drug resistance in breast cancer by inhibiting IRE1/XBP1. Oncotarget 6(38):40692–40703
Acknowledgements
This result here is based upon the data generated by TCGA Research Network: http://cancergenome.nih.gov/. We gratefully acknowledge contributions from the TCGA Research Network and the specimen donors. Furthermore, we thank Lung Cancer Group of Spanish National Cancer Center for providing validation microarray data set.
Funding
This work is supported in part by the National Natural Science Foundation of China (Nos. 31071167 and 31370751), Shanghai Municipal. Commission of Health and Family Planning (Grant No. 20144Y0179), Neil Shen's Medical Research Fund to SJTU-Yale Joint Center for Biostatistics, and Shanghai Engineering Research Center Project (17DZ2251200).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12539_2018_300_MOESM1_ESM.tiff
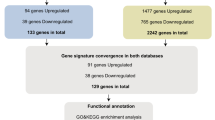
Supplementary Figure 1: Venn diagram of expressed and inhibited genes with FDR < 0.05 in LUAD and LUSC. The figure shows that inhibited gene set share larger overlap area than expressed gene set. (TIFF 2502 KB)
12539_2018_300_MOESM2_ESM.tiff
Supplementary Figure 2: Pathway enrichment on differential expression in LUAD and LUSC. A. Cell cycle and DNA replication pathway are overexpressed in LUSC. B. CAMS and immune system-related pathway are overinhibited in LUSC. C. Protein export and cell adhesion are overexpressed in LUAD. D. Wnt signaling pathway and MAPK signaling pathway and tight junction are overinhibited in LUAD. (TIFF 1781 KB)
12539_2018_300_MOESM3_ESM.tif
Supplementary Figure 3: Correlation between enriched transcription factors and target genes provided using GSEA. (TIF 4405 KB)
12539_2018_300_MOESM4_ESM.tif
Supplementary Figure 4: Correlation between XBP1, NFE2L2, and their potential target genes through different stages in TCGA data set. (TIF 13223 KB)
Rights and permissions
About this article
Cite this article
Liu, S., Wang, X., Qin, W. et al. Transcription Factors Contribute to Differential Expression in Cellular Pathways in Lung Adenocarcinoma and Lung Squamous Cell Carcinoma. Interdiscip Sci Comput Life Sci 10, 836–847 (2018). https://doi.org/10.1007/s12539-018-0300-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12539-018-0300-9