Abstract
Background
Intramuscular vaccination is among the most common source of iatrogenic pain in infants. Vapocoolant sprays are rapid-acting alternative to topical anesthetics. They provide transient anesthesia via evaporation induced skin cooling, and reduce pain due to vaccine injection in children and adults. The objective was to compare the synergistic analgesic effect of eutectic mixture of local anesthetics (EMLA) with breastfeeding (EB group) and vapocoolant spay with breastfeeding (VB group) to that of only breastfeeding (BO group) during whole cell diptheria, pertussis and tetanus (wDPT) vaccination.
Methods
A double blind randomized controlled trial was done to include infants up to 3 months of age who came for their first wDPT vaccination. The primary outcome variable was the duration of cry after vaccination. Secondary outcome variables were Modified Facial Coding Score, Neonatal Infant Pain Scale and latency of onset of cry.
Results
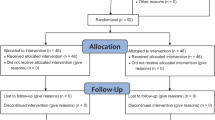
Of the 201 eligible participants, 111 babies were excluded and remaining 90 babies were randomized into three groups of thirty each. The groups did not differ significantly in baseline characteristics. Median (interquartile range, IQR) of duration of cry was lesser [35.86 (21.07-107.75) seconds] in babies receiving EMLA cream with breast feeding (EB group) and in babies receiving vapocoolant spray with breast feeding (VB group) [32.58 (21.25-106.21) seconds] as compared to babies receiving only breast feeding (BO group) [67.5 (27.6-180) seconds] (P=0.147). Difference in median (IQR) of latency of cry was not statistically significant. Modified Facial Coding Score and Neonatal Infant Pain Scale at 1 minute and 3 minutes was significantly lower in the EB and VB group, as compared to the BO group (P<0.05).
Conclusion
Addition of topical EMLA application or vapocoolant spray to breastfeeding during wDPT vaccination does not reduce duration of cry in infants up to 3 months of age. However, they are able to show reduction in pain score and further studies are warranted to assess their efficacy as pain relief measures in infants and children.
Similar content being viewed by others
References
Kennedy RM, Luhmann JD. Pharmacological management of pain and anxiety during emergency procedures in children. Paediatr Drugs 2001;3:337–354.
Iturriaga GS, Unceta-Barrenechea AA, Zárate KS, Olaechea IZ, Núñez AR, Rivero MM. Analgesic effect of breastfeeding when taking blood by heel-prick in newborns. An Pediatr (Barc). 2009;71:310–313.
Osinaike BB, Oyedeji AO, Adeoye OT, Dairo MD, Aderinto DA. Effect of breastfeeding during venepuncture in neonates. Ann Trop Paediatr 2007;27:201–205.
Efe E, Ozer ZC. The use of breast-feeding for pain relief during neonatal immunization injections. Appl Nurs Res 2007;20:10–16.
Shah PS, Aliwalas L, Shah V. Breastfeeding or breast milk to alleviate procedural pain in neonates: a systematic review. Breastfeed Med 2007;2:74–82.
Rosdahl I, Edmar B, Gisslen H, Nordin P, Lillieborg S. Curettage of molluscum contagiosum in children: analgesia by topical application of lidocaine/prilocaine cream (EMLA). Acta derm venereol 1998;68:149–153.
Taddio A, Nulman I, Goldbach M, Ipp M, Koren G. Use of lidocaine-prilocaine cream for vaccination pain in infants. J Pediatr 1994;124:643–648.
Uhari M. A eutectic mixture of lidocaine and prilocaine for alleviating vaccination pain in infants. Pediatrics 1993;92:719–721.
Abbott K, Fowler-Kerry S. The use of a topical refrigerant anesthetic to reduce injection pain in children. J Pain 1995;10:584–590.
Cohen Reis E, Holubkov R. Vapocoolant spray is equally effective as EMLA cream in reducing immunization pain in school-aged children. Pediatrics 1997;100:E5.
Maikler VE. Effects of a skin refrigerant/anesthetic and age on the pain responses of infants receiving immunizations. Res Nurs Health 1991;14:397–403.
Gupta NK, Upadhyay A, Agarwal A, Goswami G, Kumar J, Sreenivas V. Randomized controlled trial of topical EMLA and breastfeeding for reducing pain during wDPT vaccination. Eur J Pediatr 2013;172:1527–1533.
Goswami G, Upadhyay A, Gupta NK, Chaudhry R, Chawla D, Sreenivas V. Comparison of analgesic effect of direct breastfeeding, oral 25% dextrose solution and placebo during 1st DPT vaccination in healthy term infants: a randomized, placebo controlled trial. Indian Pediatr 2013;50:649–653.
Singh VB, Mishra SK, Singh T, Upadhyay A. Antinociceptive effect of exclusive breast feeding in healthy term infants during vaccination. Early Hum Dev 2008;84:50–51.
Lindh V, Wiklund U, Blomquist HK, Håkansson S. EMLA cream and oral glucose for immunization pain in 3-month-old infants. Pain 2003;104:381–388.
Mawhorter S, Daugherty L, Ford A, Hughes R, Metzger D, Easley K. Topical vapocoolant quickly and effectively reduces vaccine-associated pain: results of a randomized, single-blinded, placebo-controlled study. J Travel Med 2004;11:267–272.
Farion KJ, Splinter KL, Newhook K, Gaboury I, Splinter WM. The effect of vapocoolant spray on pain due to intravenous cannulation in children: a randomized controlled trial. CMAJ 2008;179:31–36.
Hijazi R, Taylor D, Richardson J. Effect of topical alkane vapocoolant spray on pain with intravenous cannulation in patients in emergency departments: randomised double blind placebo controlled trial. BMJ 2009;338:b215.
Costello M, Ramundo M, Christopher NC, Powell KR. Ethyl vinyl chloride vapocoolant spray fails to decrease pain associated with intravenous cannulation in children. Clin Pediatr 2006;45:628–632.
Ramsook C, Kozinetz CA, Moro-Sutherland D. Efficacy of ethyl chloride as a local anesthetic for venipuncture and intravenous cannula insertion in a pediatric emergency department. Pediatr Emerg Care 2001;17:341–343.
Upadhyay A, Aggarwal R, Narayan S, Joshi M, Paul VK, Deorari AK. Analgesic effect of expressed breast milk in procedural pain in term neonates: a randomized, placebo-controlled, double-blind trial. Acta Paediatr 2004;93:518–522.
Kataria M, Narang S, Chawla D, Sood S, Gupta PC. Oral dextrose for pain management during laser treatment of retinopathy of prematurity under topical anesthesia. Indian J Pediatr 2015;82:694–697.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupta, N.K., Upadhyay, A., Dwivedi, A.K. et al. Randomized controlled trial of topical EMLA and vapocoolant spray for reducing pain during wDPT vaccination. World J Pediatr 13, 236–241 (2017). https://doi.org/10.1007/s12519-017-0004-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12519-017-0004-y