Abstract
Background
High-mobility group box-1 (HMGB1) protein acts as an important pro-infl ammatory mediator, which is capable of activating inflammation and tissue repair. HMGB1 can bind to its receptor such as advanced glycation end products (RAGE). RAGE, in turn, can promote the production of pro-inflammatory cytokines. Soluble RAGE (sRAGE) is a truncated form of the receptor comprising the extracellular domain of RAGE and can inhibit RAGE-activation. The objective of this study was to investigate whether HMGB1 and RAGE are involved in the development of brain injury in preterm infants.
Methods
In total, 108 infants ≤34 weeks gestation at birth were divided into 3 groups according to cranial altrasound scan: mild brain damage (n=33), severe brain damage (n=8) and no brain damage (n=67). All the placentas were submitted for pathologic evaluation. Histological chorioamnionitis (HCA) was defined as neutrophil infi ltration of amniotic membranes, umbilical cord or chorionic plate. Expressions of HMGB1 and RAGE proteins were assessed by immunohistochemical analysis. The concentration of HMGB1 and sRAGE in umbilical cord blood were measured by enzyme-linked immunosorbent assay.
Results
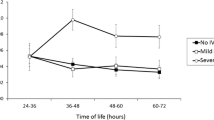
The frequency of HCA was 30.12%. HCA was associated with elevated concentrations of HMGB1 and decreased sRAGE in umbilical cord blood. The severe brain injury group demonstrated higher cord blood HMGB1 concentrations (P<0.001) and lower sRAGE concentrations (P<0.001) than both other groups. Brain injury in the premature infants was linked to intense staining for HMGB1/RAGE, particularly in infl ammatory cells.
Conclusions
Changes of cord blood HMGB1 and sRAGE of premature infants had direct relationship with the degree of infl ammation and severity of brain damage. Monitoring sRAGE and HMGB1 levels may be helpful to predict intrauterine infection and brain injury in premature infants.
Similar content being viewed by others
References
Arpino C, D’Argenzio L, Ticconi C, Di Paolo A, Stellin V, Lopez L, et al. Brain damage in preterm infants: etiological pathways. Ann 1st Super Sanita 2005;41:229–237.
Zhao J, Chen Y, Xu Y. Effect of intrauterine infection on brain development and injury. Int J Dev Neurosci 2013;31:543–549.
Edwards AD, Tan S. Perinatal infections, prematurity and brain injury. Curr Opin Pediatr 2006;18:119–124.
Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res 1997;42:1–8.
Nelson KB, Grether JK, Dambrosia JM, Walsh E, Kohler S, Satyanarayana G, et al. Neonatal cytokines and cerebral palsy in very preterm infants. Pediatr Res 2003;53:600–607.
Kaukola T, Satyaraj E, Patel DD, Tchernev VT, Grimwade BG, Kingsmore SF, et al. Cerebral palsy is characterized by protein mediators in cord serum. Ann Neurol 2004;55:186–194.
Minagawa K, Tsuji Y, Ueda H, Koyama K, Tanizawa K, Okamura H, et al. Possible correlation between high levels of IL-18 in the cord blood of pre-term infants and neonatal development of periventricular leukomalacia and cerebral palsy. Cytokine 2002;17:164–170.
Müller S, Scaffidi P, Degryse B, Bonaldi T, Ronfani L, Agresti A, et al. New EMBO members’ review: the double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J 2001;20:4337–4340.
Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 2007;81:1–5.
Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep 2004;5:825–830.
Raucci A, Palumbo R, Bianchi ME. HMGB1: a signal of necrosis. Autoimmunity 2007;40:285–289.
Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, Savitz SI, et al. Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab 2008;28:927–938.
Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med 2007;204:2913–2923.
Ivanov S, Dragoi AM, Wang X, Dallacosta C, Louten J, Musco G, et al. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood 2007;110:1970–1981.
Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol 2007;8:487–496.
Sims GP, Rowe DC, Rietdijk ST. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol 2010;28:367–388.
Dumitriu IE, Baruah P, Bianchi ME. Requirement of HMGB1 and RAGE for the maturation of human plasmacytoid dendritic cells. Eur J Immunol 2005;35:2184–2190.
Castiglioni A, Canti V, Rovere-Querini P, Manfredi AA. Highmobility group box 1 (HMGB1) as a master regulator of innate immunity. Cell Tissue Res 2011;343:189–199.
Rauvala H, Rouhiainen A. Physiological and pathophysiological outcomes of the interactions of HMGB1 with cell surface receptors. Biochim Biophys Acta 2010;1799:164–170.
De Mori R, Straino S, Di Carlo A, Mangoni A, Pompilio G, Palumbo R, et al. Multiple effects of high mobility group box protein 1 in skeletal muscle regeneration. Arterioscler Thromb Vasc Biol 2007;27:2377–2383.
Chavakis E, Hain A, Vinci M, Carmona G, Bianchi ME, Vajkoczy P, et al. High-mobility group box 1 activates integrindependent homing of endothelial progenitor cells. Circ Res 2007;100:204–212.
Czura CJ, Tracey KJ. Targeting high mobility group box 1 as a late acting mediator of inflammation. Crit Care Med 2003;31:S46–S50.
Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao L, et al. HMGB1 in health and disease. Mol Aspects Med 2014;40:1–116.
Wang H, Ward MF, Sama AE. Novel HMGB1-inhibiting therapeutic agents for experimental sepsis. Shock 2009;32:348–357.
Rouhiainen A, Kuja-Panula J, Tumova S, Rauvala H. RAGEmediated cell signaling. Methods Mol Biol 2013;96:239–263.
Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligand. BBA-Mol Cell Res. 2000;1498:99–111.
Papile L, Burstein J, Burstein R, Koffler H. Incidence and evaluation of subependymal haemorrhage: a study of children with a birthweight less than 1500 g. J Pediatr 1978;92:529–534.
De Vries LS, Eken P, Dubowitz LMS. The spectrum of leukomalacia using cranial ultrasound. Behav Brain Res 1992;49:1–6.
Chang LW, Liu J, Li WB. A preliminary study on diagnosis and grading of hypoxic-ischemic brain damage of premature infants. Zhongguo Dang Dai Er Ke Za Zhi 2007;9:293–296. [In Chinese]
Rogers BB, Alexander JM, Head J. Umbilical vein interleukin-6 levels correlate with the severity of placental inflammation and gestational age. Hum Pathol 2002;33:335–340.
Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol 1995;172:960–970.
Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. BJOG 2005;112 Suppl 1:16–18.
Ambalavanan N, Carlo WA, McDonald SA, Das A, Schendel DE, Thorsen P, et al. Cytokines and posthemorrhagic ventricular dilation in premature infants. Am J Perinatol 2012;29:731–740.
Bellussi LM, Iosif C, Sarafoleanu C, Jianu E, Duda R, Panaitescu E, et al. Are HMGB1 protein expression and secretion markers of upper airways inflammatory diseases? J Biol Regul Homeost Agents 2013;27:791–804.
Higgins SJ, Xing K, Kim H, Kain DC, Wang F, Dhabangi A, et al. Systemic release of high mobility group box 1 (HMGB1) protein is associated with severe and fatal Plasmodium falciparum malaria. Malar J 2013;12:105.
Allonso D, Belgrano FS, Calzada N, Guzmán MG, Vázquez S, Mohana-Borges R. Elevated serum levels of high mobility group box 1 (HMGB1) protein in dengue-infected patients are associated with disease symptoms and secondary infection. J Clin Virol 2012;55:214–219.
Goova MT, Li J, Kislinger T. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am J Pathol 2001;159:513–525.
Buhimschi CS, Baumbusch MA, Dulay AT, Oliver EA, Lee S, Zhao G, et al. Characterization of RAGE, HMGB1, and S100B in inflammation-induced preterm birth and fetal tissue injury. Am J Pathol 2009;175:958–975.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, HY., Ma, JL., Shan, JY. et al. High-mobility group box-1 and receptor for advanced glycation end products in preterm infants with brain injury. World J Pediatr 13, 228–235 (2017). https://doi.org/10.1007/s12519-016-0077-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12519-016-0077-z