Abstract
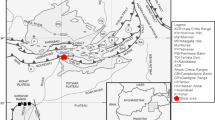
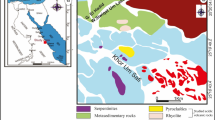
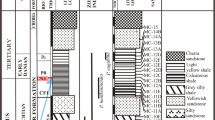
In this research, Cretaceous claystones in the Az Zabirah area, Northern Saudi Arabia were evaluated to investigate the paleoenvironmental and paleoclimatic conditions during deposition of the claystone sediments. Outcrop samples of claystone from the bauxite zone profile in the Az Zabirah area were analyzed using multi-proxy geochemical and coupled with palynological analysis. Palynological analysis suggested that the age of the Az Zabirah claystones is Maastrichtian of Upper Cretaceous. This evidence is valid due to the presence of microspore taxa Gabonisporis sp. The Az Zabirah claystones have abundant angiosperm taxa, indicating a large terrestrial influx in the interval of the claystone. This finding is confirmed from geochemistry of major and trace elements and mineral compositions. The high concentration of terrestrial detritus oxides, such as SiO2, Al2O3, and TiO2, infers that the Az Zabirah claystones were sourced from terrigenous origin. This is consistent with a significant amount of quartz and kaolinite in the Az Zabirah claystones. The claystones could be deposited under oxic paleo-redox and warm-humid with little aridity climatic conditions. This is indicated by their concentration of the trace element concentrations along with their geochemical ratios. Warm-humid climate condition is also largely supported by the presence of a significant amount of kaolinite.












Similar content being viewed by others
References
Al-Bassam K (2005) Mineralogy and geochemistry of the Hussainiat karst bauxite and Zabira stratiform bauxite in Northern Arabian Peninsula. Iraqi Bulletin of Geology and Mining 1:15–44
Al-men WF Von, 1982. Review of palynology reports, Az Zabirah, Oct., Arabian American Oil Company, Dhahran
Al-men WF Von, 1983. Palynological report—Az Zabirah samples. Aug., Arabian American Oil Company, Dhahran
Al-Mesbah M., 2009. Az Zabirah central zone project—preliminary technical and economical research. Report. hal-00595764
Bardossy G (1982) Karst bauxites. Elsevier, Amsterdam, p 441
Barwise AJG (1990) Role of nickel and vanadium in petroleum classification. Energy and Fuels 4(6):647–652. https://doi.org/10.1021/ef00024a005
Bechtel A, Gratzer R, Sachsenhofer RF (2001) Chemical characteristics of Upper Cretaceous (Turonian) jet of the Gosau Group of Gams/Hieflau (Styria, Austria). Int J Coal Geol 46(1):27–49. https://doi.org/10.1016/S0166-5162(01)00007-6
Beckmann B, Flögel S, Hofmann P, Schulz M, Wagner T (2005) Orbital forcing of Cretaceous river discharge in tropical Africa and ocean response. Nature 437(7056):241–244. https://doi.org/10.1038/nature03976
Berner RA, Raiswell R (1983) Burial of organic carbon and pyrite sulfur in sediments over Phanerozoic time: a new theory. Geochim Cosmochim Acta 47(5):855–862. https://doi.org/10.1016/0016-7037(83)90151-5
Black, R.Y., Bognar, B., Watson, A.D., Barnes, D.P., 1982. Evaluation of the Az Zabira bauxite deposit (1980–1982). Riofinex Ltd., Tech. Rep
Bowden RA, 1981 Geology of the Az Zabira bauxite occurrence. Riofinex Ltd., open file report
Calvert SE, Bustin RM, Ingall ED (1996) Influence of water column anoxia and sediment supply on the burial and preservation of organic carbon in marine shales. Geochim Cosmochim Acta 60(9):1577–1593. https://doi.org/10.1016/0016-7037(96)00041-5
Chamley H (1989) Clay sedimentology. Springer, Berlin, p 623. https://doi.org/10.1007/978-3-642-85916-8
Deng HW, Qian K (1993) Analysis on sedimentary geochemistry and environment. Science Technology Press, Gansu, pp 15–85 (in Chinese)
Durand B, Nicaise G (1980) Procedures of kerogen isolation. In: Durand B (ed) Kerogen, insoluble organic matter from sedimentary rocks. Éditions Technip, Paris, pp 35–53
Ercegovac M, Jeremi CM, Djaji CS (1997) Miocene sedimentary organic facies and palynofacies in Drmno depression (Serbia). Ann Geol Penins Balk 61(1):143e165
Fauconnier D, (1981) Etude Palynologique d ‘une Serie d ‘EchantilIons Associes aux Formations Bauxitiques. SGN GEO 474, BRGM, Orleans
Fauconnier D, (1982) Etude Palynologique de 13 Echantillons. 82 GEO EM 07, BRG!, Orleans
Hieronymus B, Kotschoubey B, Boulegue J (2001) Gallium behavior in some contrasting lateritic profiles from Cameroon and Brazil. J Geochem Explor 72(2):147–163. https://doi.org/10.1016/S0375-6742(01)00160-1
Jahren AH (2007) The Arctic forest of the Middle Eocene. Annu Rev Earth Planet Sci 35(1):509–540. https://doi.org/10.1146/annurev.earth.35.031306.140125
Jia J, Bechtel A, Liu Z, Susanne AI, Strobl PS, Reinhard FS (2013) Oil shale formation in the Upper Cretaceous Nenjiang Formation of the Songliao Basin (NE China): implications from organic and inorganic geochemical analyses. Int J Coal Geol 113:11–26. https://doi.org/10.1016/j.coal.2013.03.004
Jones B, Manning D (1994) Comparison of geochemical indices used for the interpretation of palaeo redox conditions in ancient mudstones. Chem Geol 111(1-4):111–129. https://doi.org/10.1016/0009-2541(94)90085-X
Karadag MM, Kupeli MS, Aryk F, Ayhan A, Zedef V, Döyen A (2009) Rare earth elements (REE) geochemistry and genetic implications of the Mortaş bauxite deposit (Sydişehir/Konya-Southern Turkey). Chem Erde 69(2):143–159. https://doi.org/10.1016/j.chemer.2008.04.005
Larsson LM, Vajda V, Rasmussen ES (2006) Early Miocene pollen and spores from western Jylland, Denmark—environmental and climatic implications. GFF 128(3):261–272. https://doi.org/10.1080/11035890601283261
Laville P, (1982) Textural research of bauxite-profiles from Az Zabira District (NE Saudi Arabia). BRGM report
Lerman A (1989) Lakes: chemistry, geology, physics. Geological Press, Beijing, pp 10–100 (in Chinese)
Liu YJ, Cao LM, Li ZL, Wang HN, Chu TQ, Zhang JR (1984) Element geochemistry, Science Press, Beijing, pp 283–372 (in Chinese)
Mohialdeen IMJ, Raza SM (2013) Inorganic geochemical evidence for the depositional facies associations of the Upper Jurassic Chia Gara Formation in NE Iraq. Arab. J Geosci 6:4755–4770
Nesbitt HW, Young GM, McLennan SM, Keays RR (1996) Effects of chemical weathering and sorting on the petrogenesis of siliciclastic sediments, with implications for provenance studies. J Geol 104(5):525–542. https://doi.org/10.1086/629850
Nichols DJ, Pocknall DT, (1994). Relationships of palynofacies to coal-depositional environments in the upper Paleocene of the Gulf Coast Basin, Texas, and the Powder River Basin, Montana and Wyoming. Sedimentation of organic particles. Cambridge University Press. pp. 217-238
Oboh-Ikuenobea FE, Spencera MK, Campbellb CE, Haselwandera RD (2012) Palynology. Special issue in honor of Douglas J. Nichols. Volume 36, Supplement 1, pp. 63–79
Pattan JN, Pearce NJG (2009) Bottom water oxygenation history in south-eastern Arabian Sea during the past 140 ka: results from redox-sensitive elements. Palaeogeography Paleoclimate Palaeoecology 280(3-4):396–405. https://doi.org/10.1016/j.palaeo.2009.06.027
Powers RW, Ramirez LF, Redmond CD, Elberg EL Jr, 1966. Geology of the Arabian Peninsula: sedimentary geology of Saudi Arabia. US Geol. Surv. Prof. Paper, 560-D, p. 147
Premoli-Silva I, 1982. Paleontological report. July, University of Milan
Ratcliffe KT, Wright AM, Hallsworth C, Morton A, Zaitlin BA, Potocki D, Wray DS (2004) Alternative correlation techniques in the petroleum industry: an example from the (Lower Cretaceous) BasalQuartz, Southern Alberta. Bullee Am Assoc Pet Geol 88:419–432
Ratcliffe KT, Wright AM, Montgomery P, Palfrey A, Vonk A, Vermeulen J, Barrett M, (2010) Application of chemostratigraphy to the Mungaroo Formation, the orgoneld, offshore Northwest Australia. Australian Petroleum Production and Exploration Association Journal (50th Anniversary Issue), 371–388
Reimann C, de Caritat P, (1998). Chemical elements in the environment. Springer-Verlag, New York, Inc., New York (397 pp.)
Rieu R, Allen PA, Plotze M, Pettke T (2007) Compositional and mineralogical variations in a Neoproterozoic glacially influenced succession, Mirbat area, south Oman: implications for paleoweathering conditions. Precambrian Res 154(3-4):248–265. https://doi.org/10.1016/j.precamres.2007.01.003
Ross DJK, Bustin RM (2009) Investigating the use of sedimentary geochemical proxies for palaeoenvironment interpretation of thermally mature organic-rich strata: examples from the Devonian–Mississippian shales, Western Canadian Sedimentary Basin. Chem Geol 260(1-2):1–19. https://doi.org/10.1016/j.chemgeo.2008.10.027
Saxby JD (1970) Isolation of kerogen in sediments by chemical methods. Chem Geol 6:173–184. https://doi.org/10.1016/0009-2541(70)90017-3
Stricto PS, Lato S (2015) Palynology (pollen, spores, etc.). Springer, Netherlands
Suttner LJ, Dutta PK (1986) Alluvial sandstone composition and palaeoclimate. 1. Framework mineralogy. J Sediment Petrol 56:329–345
Urban NR, Ernst K, Bernasconi SM (1999) Addition of sulfur to organic matter during early diagenesis of lake sediments. Geochim Cosmochim Acta 63(6):837–853. https://doi.org/10.1016/S0016-7037(98)00306-8
Wang AH (1996) Discriminant effect of sedimentary environment by the Sr/Ba ratio of different existing forms. Acta Sedimentol Sin 14:168–173
Wang JS, Huang XZ, Sui JC, Shao HS, Yan CF, Wang SQ, He ZR (1997) Evolutional characteristics and their paleoclimate significance of trace elements in the Hetaoyuan Formation, Biyang depression. Acta Sedimentol Sin 15:65–70 (in Chinese with English abstract)
Wenger LM, Isaksen GH (2002) Controls of hydrocarbon seepage intensity on level of biodegradation in sea bottom sediments. Org Geochem 12:1277–1292
Yan D, Chen D, Wang Q, Wang J (2010) Large-scale climatic fluctuations in the latest Ordovician on the Yangtze block, South China. Geology 38(7):599–602. https://doi.org/10.1130/G30961.1
Acknowledgements
The authors wish to acknowledge the financial support by the King Saud University Research Grant, Deanship of Scientific Research, College of Science Research Center. The constructive comments and suggestions by Dr. Ramadan Abu-Zied that improved the revised manuscript are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yahya, M.M., Hakimi, M.H., Galmed, M.A. et al. Paleoenvironmental and paleoclimatic conditions during the deposition of the bauxite layer (Upper Cretaceous) using multi-proxy geochemical and palynological analyses, in the Zabirah Area, Northern Saudi Arabia. Arab J Geosci 11, 15 (2018). https://doi.org/10.1007/s12517-017-3379-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-017-3379-0