Abstract
This study investigated the mechanisms involved in removing arsenic from water using zero-valent iron (ZVI) as sorbent. Relatively limited information is available on the kinetics aspects of sorption of arsenic compounds onto ZVI. In order to gain an understanding of the sorption kinetics, a detailed study was conducted in a controlled batch test and developed sorption kinetic model.
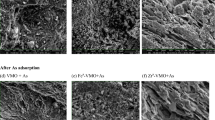
The effects of different arsenic concentrations on the kinetics sorption rates of arsenic(V) and arsenic(III) were investigated. Arsenic(V) was removed by two mechanisms—surface adsorption and co-precipitation with Fe(III) on ZVI, while arsenic(III) was removed by adsorption on ZVI and oxidized to arsenic(V). Reaction rate constants were calculated for arsenic(V) and arsenic(III) at different concentrations by a second-order kinetic model.
The results indicate that ZVI could be employed as sorbent materials to enhance the adsorption and co-precipitation processes to improve the removal rate of arsenic from water. The results also showed that the arsenic(III) oxidized to arsenic(V), while the analyses indicated that there was no measurable reduction of arsenic(V) to arsenic(III).
Similar content being viewed by others
References
Banerjee K, Amy GL, Prevost M, Nour S, Jekel M, Gallagher PM, Blumenschein CD (2008) Kinetic and thermodynamic aspects of adsorption of arsenic onto granular ferric hydroxide (GFH). Water Res 42:3371–3378
Bang S, Johnson MD, Korfiatis GP, Meng X (2005) Chemical reactions between arsenic and zero-valent iron in water. Water Res 39:763–770
Cho HH, Park JW (2006) Sorption and reduction of tetrachloroethylene with zero-valent iron and amphiphilic molecules. Chemosphere 64:1047–1052
Farrell J, Wang J, O’Day P, Conklin M (2001) Electrochemical and spectroscopy study of arsenate removal from water using zero-valent iron media. Environ Sci Technol 35:2026–2032
Ferguson JG, Anderson MA (1974) Chemical form of arsenic in water supplies and their removal. In: Rubin AJ (ed) Chemistry of water supply, treatment, and distribution, pp 137–158. Ann Arbor Science, Ann Arbor
Fields K, Chen A, Wang L (2000) Arsenic removal from drinking water by iron removal plants. Batelle EPA/600/R-00/086
Fuller CC, Davis JA, Waychunas GA (1993) Surface chemistry of ferrihydrite, part 2: kinetics of arsenate adsorption and co-precipitation. Geochim Cosmochim Acta 57:2271–2282
Grossl PR, Eick M, Sparks DL, Goldberg S, Anisworth CC (1997) Arsenate and chromate retention mechanism on geothite, 2: kinetic evaluation using pressure-jump relaxation technique. Environ Sci Technol 31:321–326
Johnston RB, Singer PC (2007) Redox reactions in the Fe–As–O2 system. Chemosphere 69:517–525
Kouznetsova I, Bayer P, Ebert M, Finkel M (2007) Modelling the long-term performance of zero-valent iron using a spatiotemporal approach for iron aging. J Contam Hydrol 90:58–80
Kundu S, Gupta AK (2005) Sorption kinetics of As(V) with iron-oxide-coated cement—a new adsorbent and its application in the removal of arsenic from real-life groundwater samples. J Environ Sci Health, Part A 40:2227–2246
Lackovic JA, Nikolaidis NP, Dobbs GM (2000) Inorganic arsenic removal by zero-valent iron. Environ Eng Sci 17:29–39
Leupin OX, Hug SJ (2005) Oxidation and removal of arsenic(III) from aerated groundwater by filtration through sand and zero-valent iron. Water Res 39:1729–1740
Lin YT, Weng CH, Chen FY (2008) Effective removal of AB24 dye by nano/micro-size zero-valent iron. Sep Purif Technol 64:26–30
Melitas N, Wang JP, Conklin M, O’Day P, Farrell J (2002) Understanding soluble arsenate removal kinetics by zero-valent iron. Environ Sci Technol 36:2074–2081
Mu Y, Yu HQ, Zheng JC, Zhang SJ, Sheng GP (2004) Reductive degradation of nitrobenzene in aqueous solution by zero-valent iron. Chemosphere 54:789–794
Nikolaidis NP, Dobbs GM, Lackovic JA (2003) Arsenic removal by zero-valent iron: field, laboratory and modeling studies. Water Res 37:1417–1425
Nordstrom DK, Alpers CN (1999) Negative pH, efflorescent mineralogy, and consequences for environmental restoration at the Iron Mountain Superfund Site, CA. Proc Natl Acad Sci USA 96:3455–3462
Pontius FW, Brown GK, Chien JC (1994) Health implications of arsenic in drinking water. J Am Water Works Assoc 86(9):52–63
Raven KP, Jain A, Loeppert RH (1998) Arsenite and arsenate adsorption on ferrihydrite: kinetics, equilibrium, and adsorption envelope. Environ Sci Technol 32:344–349
Sasaki K, Nakano H, Wilopo W, Miura Y, Hirajima T (2008) Sorption and speciation of arsenic by zero-valent iron. Colloids Surf A, Physicochem Eng Asp 347:8–17
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Smith AH, Hopenhayn-Rich C, Bates MN, Goeden HM, Hertz-Picciotto I, Duggan R, Wood HM, Kosnett, Smith MT (1992) Cancer risks from arsenic in drinking water. Environ Health Perspect 97:259–267
Su C, Puls R (2001) Arsenate and arsenite removal by zero-valent iron: kinetics, redox transformation and implications for in situ groundwater remediation. Environ Sci Technol 35:1487–1492
Triszcz JM, Porta A, Einschlag FG (2009) Effect of operating conditions on iron corrosion rates in zero-valent iron systems for arsenic removal. Chem Eng J 150:431–439
WHO (1993) Guidelines for drinking-water quality, vol 1: recommendations, 2nd edn. WHO, Geneva
Zhang O, Pan B, Zhang W, Pan BI, Zhang Q, Ren H (2008) Arsenate removal from aqueous media by nano-sized hydrated ferric oxide (HFO)-loaded polymeric sorbents: effect of HFO loadings. Ind Eng Chem Res 47:3957–3962
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eljamal, O., Sasaki, K., Tsuruyama, S. et al. Kinetic Model of Arsenic Sorption onto Zero-Valent Iron (ZVI). Water Qual Expo Health 2, 125–132 (2011). https://doi.org/10.1007/s12403-010-0030-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-010-0030-7