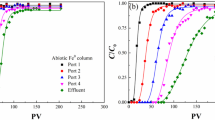
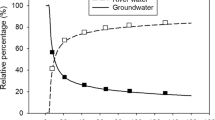
Abstract
A mathematical model is developed to simulate the reduction processes and precipitation of Fe(OH)3 and FeS in an unconfined coastal aquifer. The available measured data of the selected aquifer shows that the saltwater region of the coastal aquifer is highly reduced. Mn2+, Fe2+ and HS− have been formed in the reduced saltwater region of the aquifer as a result of the bacteria-mediated reduction of MnO2, Fe(OH)3 and SO \(_{4}^{\ \ 2-}\) respectively. Formed Fe2+ is oxidised again when it reaches the oxygen available mixing zone. Moreover, the formed Fe2+ and HS− precipitate as FeS in the reduced saltwater region. The objective of the present study is to simulate the above-mentioned reduction and oxidation processes by coupling solute transport, bacteria-mediated reduction and precipitation together. Bacterial growth is assumed to follow double Monod kinetic equation. Four bacterial groups (X 1, X 2, X 3 and X 4) are described. Bacteria group X 1 uses oxygen under aerobic conditions and NO \(_{3}^{\ \ -}\) under anaerobic conditions as electron acceptor. Anaerobic bacterial groups X 2, X 3 and X 4 use respectively MnO2, Fe(OH)3 and SO \(_{4}^{\ \ 2-}\) as electron acceptors. Organic carbon which behaves as the electron donor is considered as the most important factor for the bacteria-mediated reduction processes. Numerical results are presented for ten years of calculation and are discussed highlighting the importance of more detailed studies on the biogeochemical aspects of coastal aquifers and their numerical simulations.
Similar content being viewed by others
References
Akagi K, Hosokawa T, Hiroshiro Y, Jinno K (2006) Modelling of physical and geological behaviours of saltwater in a coastal aquifer. Adv Geosci 4:251–260
Andersen PF, Mercer JW, White HO (1998) Numerical modelling of seawater intrusion at Hallandale, Florida. Groundwater 26:619–630
Appelo CAJ, Postma D (2007) Geochemistry groundwater and pollution, 2nd edn. AA Balkema Publishers, Leiden
Baedecker MJ, Cozzarelli IM, Eganhouse RP, Siegei DI, Benett PC (1993) Crude oil in a shallow sand gravel aquifer, biogeochemical reactions and mass balance modelling in anoxic groundwater. J Appl Geochem 8:569–586
Bjerg PL, Rugge K, Pedersen JK, Christensen TH (1995) Distribution of redox-sensitive groundwater quality parameters down gradient of a landfill (Grindsted, Denmark). Environ Sci Technol 29:1387–1394
Chapelle FH, Lovley DR (1992) Competitive exclusion of sulfate reduction by Fe(III)-reducing bacteria: a mechanism for producing discrete zones of high-iron ground water. Groundwater 30(1):29–36
Chapelle FH, Haak SK, Adriaens P, Henry MA, Bradley PM (1996) Comparison of Eh and H2 measurements for delineating redox processes in a contaminated aquifer. Environ Sci Technol 30:3565–3569
Chapelle FH (2001) Ground-water microbiology and geochemistry, 2nd edn. Wiley, New York
Charette MA, Busseler KO, Andrews JE (2001) Utility of radium isotopes for evaluating the input and transport of groundwater-derived nitrogen to a Cape Cod estuary. Limnol Oceanogr 46(2):465–470
Chiang CY, Dawson CN, Wheeler MF (1991) Modelling of in situ bio restoration of organic compounds in groundwater. Transp Porous Media 6:667–702
Christensen TH, Bjerg PL, Banwart SA, Jakobsen R, Heron G, Albrechtsen HJ (2000) Characterization of redox conditions in groundwater contaminant plumes. J Contam Hydrol 45:165–241
Eljamal O, Jinno K, Hosokawa T (2008) Development of biological treatment model with biological clogging processes in porous media: model application to a column study. Jpn Assoc Groundwater Hydrol J 50(4):275–290
Essink GHPO (2001) Density dependent groundwater flow. Academic Publishing of Utrecht University, Utrecht
Ghassemi F, Chen TH, Jakeman AJ, Jacobson G (1993) Two and three-dimensional simulation of seawater intrusion: performances of the “SUTRA” and HST3D” models. AGSO J Austr Geol Geophys 14(2–3):219–226
Guerra G, Jinno K, Hiroshiro Y, Nakamura K (2004) The multi-component solute transport model with cation exchange under redox environment and its application for designing the slow infiltration set-up. Mem Eng Kyushu Univ 64(1):79–100
Hiroshiro Y, Jinno K, Berndtsson R (2006) Hydro-geochemical properties of a salinity-affected coastal aquifer in western Japan. Hydrol Process 20:1425–1435
Hockin SL, Gadd GM (2003) Linked redox precipitation of sulfur and selenium under anaerobic conditions by sulfate-reducing bacterial biofilms. Appl Environ Microbiol 69(12):7063–7072
Hove M, Van HRP, Lewis AE, (2007) Iron solids formed from oxidation precipitation of ferrous sulfate solutions. AIChE J 53(10):2569–2577
Hunter KS, Wang Y, Cappellen PV (1998) Kinetic modelling of microbially-driven redox chemistry of subsurface environments: coupling transport, microbial metabolism and geochemistry. J Hydrol 209:53–80
Huyakorn PS, Pinder GF (1983) Computational method in subsurface flow. Academic Press, New York
Jakobsen R, Postma D (1999) Redox zoning, rates of sulfate reduction and interactions with Fe-reduction and methanogenesis in a shallow sandy aquifer, Romo, Denmark. Geochim Cosmochim Acta 63(1):137–151
Jinno K, Hosokawa T, Hiroshiro Y, Ohgushi M (2001) Mixing of fresh and salt groundwater in a sandy beach using pipe draining to extend the unsaturated zone. In: Proceedings of the 3rd international conference on future groundwater resources at risk (Lisbon, Portugal), pp 641–648
Jinno K, Akagi K, Hiroshiro Y, Hosokawa T, Yasumoto J (2007) Geochemical processes and their modelling at the fresh and salt water mixing zone. Proc ModelCARE 2007, Denmark, IAH Publ 320:191–196
Kinzelbach W, Schäfer W (1994) Modelling and design of in situ bioremediation measures. IAHS 220:399–412
Lensing HJ, Vogt M, Herring B (1994) Modelling of biologically mediated redox processes in the subsurface. J Hydrol 159:125–143
Murphy EM, Ginn TR (2000) Modelling microbial processes in porous media. Hydrogeol J 8:142–158
Nakagawa K, Hosokawa T, Iwamitsu K, Hiroshiro Y, Jinno K (2002) Study of the mixing of fresh and salt groundwater in a sandy beach using pipe drains to extend the unsaturated zone. J Hydraul Eng JSCE 46:181–186
Rickard D (1995) Kinetics of FeS precipitation: Part 1. Competing reaction mechanisms. Geochim Cosmochim Acta 59(21):4367–4379
Rittmann BE, McCarty PL (2001) Environmental biotechnology: principles and applications. McGraw-Hill, New York
Rivera A, Ledoux E, Sauvagna S (1990) A compatible single-phase/two-phase numerical model: 2. Application to a coastal aquifer in Mexico. Groundwater 28(2):215–223
Schäfer D, Schäfer W, Kinzelbach W (1998a) Simulation of reactive processes related to biodegradation in aquifers: 1. Structure of the three-dimensional reactive transport model. J Contam Hydrol 31:167–186
Schäfer D, Schäfer W, Kinzelbach W (1998b) Simulation of reactive processes related to biodegradation in aquifers: 2. Model application to a column study on organic carbon degradation. J Contam Hydrol 31:187–209
Snyder M, Taillefert M, Ruppel C (2004) Redox zonation at the saline-influenced boundaries of a permeable surfacial aquifer: effects of physical forcing on the biogeochemical cycling of iron and manganese. J Hydrol 296:164–178
Tobias CR, Macko SA, Anderseon IC, Canuel EA, Harvey JW (2001) Tracking the fate of a high concentration groundwater nitrate plume through a fringing marsh: a combined groundwater tracer and in situ isotope enrichment study. Limnol Oceanogr 46(8):1977–1989
White DC, Fredrickson JF, Gehron MH, Smith GA, Martz RF (1983) The groundwater aquifer microbiota: biomass, community structure, and nutritional status. Dev Ind Microbiol 24:189–199
Yamaguchi T, Yamazaki S, Uemura S, Tseng IC, Ohashi A, Harada H (2001) Microbial-ecological significance of sulphide precipitation within anaerobic granular sludge revealed by micro-electrodes study. Water Resourc 35(14):3411–3417
Zhou X, Ju CX, Ning X, Wang J (2000) Numerical simulation of seawater intrusion near Beihai, China. Environ Geol 40(1–2):223–233
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perera, E.D.P., Jinno, K. & Hiroshiro, Y. Bacteria-mediated Reduction and Precipitation of Fe(OH)3 and FeS in the Subsurface of a Coastal Aquifer: A Numerical Investigation. Water Qual Expo Health 2, 15–30 (2010). https://doi.org/10.1007/s12403-009-0021-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-009-0021-8