Abstract
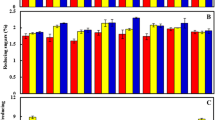
Enhancing cane and sugar productivity is the major concern of sugarcane growing countries. The present study was aimed to monitor the expression of SAI gene related to the sugar content during elongation phase and its modulation at the onset of cane ripening using an early maturing, high-sugar genotype (CoJ 64) and a mid-late maturing, low-sugar genotype (BO 91). The results indicated comparatively higher concentrations of non-reducing, total sugars and non-reducing/reducing sugar ratio (NRS/RS ratio) in CoJ 64 in all plant tissues (root, stalk and leaf); the highest was in stalk tissue. Similar to sugar content, Brix value (a field indicator of sucrose content) was also higher in CoJ 64. In contrast to this, reducing sugars content, SAI enzyme activity and gene expression level were relatively higher in BO 91. Root tissues showed higher SAI gene expression in BO 91 as compare to CoJ 64, indicating its utility as a molecular tool for characterizing high- and low-sugar genotypes at an early growth stage. Correlation data indicated a negative association of SAI activity and gene expression with non-reducing sugars and NRS/RS ratio, but positive with reducing sugars contents. Differential SAI expression in low- and high-sugar genotypes at the stage of crop elongation may help to manipulate sucrose accumulation process in low-sugar genotypes. In this domain, an effort was also made to improve sucrose content in cane stalk of BO 91 during inclined phase using enzyme effectors (Mg, Mn, B, Mg + Mn, ethrel and a mixture of Mg + Mn + ethrel as a chemical formulation). Chemical formulation was found to be more effective for increasing sucrose content with reduced SAI activity. Findings thus suggest downregulation of SAI activity in response to foliar application of chemical formulation vis-à-vis improvement in sucrose content might be due to significant negative association of SAI gene with non-reducing sugars (sucrose) in both low- and high-sugar genotypes.





Similar content being viewed by others
Abbreviations
- HEPES:
-
4-(2-Hydroxyethyl)-1-piperazine-N’-2-ethane sulfonicacid
- DAP:
-
Days after planting
References
Chandra, A., P.K. Verma, M.N. Islam, M.P. Grisham, R. Jain, A. Sharma, K. Roopendra, K. Singh, P. Singh, I. Verma, and S. Solomon. 2014. Expression analysis of genes associated with sucrose accumulation in sugarcane (Saccharum spp. hybrids) varieties differing in content and time of peak sucrose storage. Plant Biology 17 (3): 608–617.
Chandra, A., R. Jain, and S. Solomon. 2012. Complexities of invertase controlling sucrose accumulation and retention in sugarcane. Current Science 102: 857–866.
Chandra, A., R. Jain, R.K. Rai, and S. Solomon. 2010. Designing and application of sucrose controlling enzyme soluble acid invertase (SAI) gene based primer pairs in sugarcane. National Academy Science Letters 33: 355–359.
Eastwood, D., and H.B. Davis. 1997. Chemical ripening in Guyana: Progress and prospects. Sugarcane International 3: 4–7.
Huber, S.C., and J.L. Huber. 1996. Role and regulation of sucrose phosphate synthase in higher plants. Annual Review Plant Physiology and Plant Molecular Biology 47: 431–444.
Jain, R., A. Chandra, and S. Solomon. 2013. Impact of exogenously applied enzymes effectors on sucrose metabolizing enzymes (SPS, SS and SAI) and sucrose content in sugarcane. Sugar Tech 4: 370–378.
Jain, R., S. Solomon, Y. Rui-Li, A. Chandra, and A.K. Shrivastava. 2014. Ethephon: Impact on sugarcane physiology and sugar productivity. Agrotech publishing academy ISBN: (13) 978-81-8321-322-6. 157.
Kaur, S., S.K. Batta, J.S. Sital, K.P. Sharma, and A.P.S. Mann. 2002. Partial purification and properties of soluble invertase isoforms from sugarcane storage tissue. Indian Sugar 51: 851–857.
Koch, K.E. 1996. Carbohydrate-modulated gene expression in plants. Annual Review of Plant Physiology and Molecular Biology 47: 509–540.
Lee, M.H., C.C. Yang, J.C. Su, and P.D. Lee. 2005. Biochemical characterization of rice sucrose phosphate synthase under illumination and osmotic stress. Botanical Bulletin of Academia Sinica 46: 43–52.
Li, Y.R., and S. Solomon. 2003. Ethephon, a versatile growth regulator for sugarcane industry. Sugar Tech 5: 213–224.
Lontom, M., M. Kosittrakun, and S.E. Lingle. 2008. Relationship of acid invertase activities to sugarcane internode during ripening and after harvest. Thai Journal of Agricultural Science 41: 143–151.
Lowry, O.H., N.J. Rosebrough, A.L. Farr, and R.J. Randall. 1951. Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry 193: 265–275.
Mamet, L.D., R. Domaingue, and F.C. Nayamuth. 1996. Research and development on earliness of ripening of sugarcane (Saccharum spp.) at the Mauritius sugar industry research Institute. Proceedings of South African Sugar Technologists Association 70: 106–110.
Medtantsava, E.P., M.G. Vertlib, and G.K. Budnikov. 1998. Metal ions as enzyme effectors. Russian Chemical Reviews 67: 225–232.
Morell, M., and L. Copeland. 1985. Sucrose synthase of soybean nodules. Plant Physiology 78: 149–154.
Murata, T. 1972. Sucrose synthetase of rice grains and potato tubers. Agricultural Biological Chemistry 36: 1815–1818.
Nelson, N. 1944. A photometric adaptation of Somogyi method for the determination of glucose. Journal of Biological Chemistry 153: 375–380.
Sachdeva, M., A.P.S. Mann, and S.K. Batta. 2003. Multiple forms of soluble invertases in sugarcane juice: Kinetic and thermodynamic analysis. Sugar Tech 5: 31–35.
Solomon, S., H.N. Shahi, I. Singh, P.C. Joshi, and S. Deb. 2001a. Chemical ripening of sugarcane: Sucrose enhancing response of dinitrosocifrol and triacotanol. Sugar Tech 3: 53–54.
Solomon, S., H.N. Shahi, S.K. Duttamajumder, I. Singh, and V.K. Madan. 2001b. Effect of ethephon on sugarcane grown under subtropical climate. Proceedings of International Society for Sugar Cane Technologists 24: 177–179.
Strum, A., and G.Q. Tang. 1999. The sucrose cleaving enzymes of plants are crucial for development, growth and carbon partitioning. Trends in Plant Science 4 (10): 401–407.
Tana, B., S. Chanprame, N. Tienseree, and S. Tadakittisarn. 2014. Relationship between invertase enzyme activities and sucrose accumulation in sugarcane (Saccharum spp.). Natural Science 48: 869–879.
Venkataramana, S., K.M. Naidu, and S. Singh. 1991. Invertases and growth factors dependent sucrose accumulation in sugarcane. Plant Science 74: 65–72.
Walker, R.P., A.L. Winters, and C.J. Pollock. 1997. Purification and characterization of invertases from leaves of Lolium temulentum. The New Phytologist 135: 259–266.
Wobus, U., and H. Weber. 1999. Sugars as signal molecules in plant seed development. Biological Chemistry 380: 937–944.
Yang, L., D.B. Qing, Z.X. Na, Y.M. Qi, L. Ming, and L.G. Ving. 2013. Correlation analysis between the key enzymes activities and sugar content in sweet sorghum (Sorghum bicolor L. Moench) stems at physiological maturity stage. Australian Journal of Crop Sciences 7: 84–92.
Yao, R.L., Y.R. Li, G.R. Zhang, and L.T. Yang. 2002. Endogenous hormone levels at technical maturing stage of sugarcane. Sugar Tech 4: 14–18.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jain, R., Singh, S.P., Singh, A. et al. Soluble Acid Invertase (SAI) Activity and Gene Expression Controlling Sugar Composition in Sugarcane. Sugar Tech 19, 669–674 (2017). https://doi.org/10.1007/s12355-017-0511-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-017-0511-0