Abstract
Cardiac amyloidosis is a form of restrictive cardiomyopathy resulting in heart failure and potential risk on arrhythmia, due to amyloid infiltration of the nerve conduction system and the myocardial tissue. The prognosis in this progressive disease is poor, probably due the development of cardiac arrhythmias. Early detection of cardiac sympathetic innervation disturbances has become of major clinical interest, because its occurrence and severity limits the choice of treatment. The use of iodine-123 labelled metaiodobenzylguanidine ([I-123]MIBG), a chemical modified analogue of norepinephrine, is well established in patients with heart failure and plays an important role in evaluation of sympathetic innervation in cardiac amyloidosis. [I-123]MIBG is stored in vesicles in the sympathetic nerve terminals and is not catabolized like norepinephrine. Decreased heart-to-mediastinum ratios on late planar images and increased wash-out rates indicate cardiac sympathetic denervation and are associated with poor prognosis. Single photon emission computed tomography provides additional information and has advantages for evaluating abnormalities in regional distribution in the myocardium. [I-123]MIBG is mainly useful in patients with hereditary and wild-type ATTR cardiac amyloidosis, not in AA and AL amyloidosis. The potential role of positron emission tomography for cardiac sympathetic innervation in amyloidosis has not yet been identified.
Similar content being viewed by others
Introduction
Patients with amyloidosis are prone to developing disturbances in autonomic innervation: dysautonomia.1 Cardiac dysautonomia can be caused by amyloid infiltration into the myocardial and conduction tissue, resulting in conduction and rhythm disorders. Cardiac dysautonomia is common in patients with transthyretin-related amyloidosis (ATTR type) and in patients with immunoglobulin light chain-derived amyloidosis (AL type).2 More specific, patients with the hereditary form of ATTR type amyloidosis (hATTR, formerly called familial amyloid polyneuropathy) frequently develop polyneuropathy and dysautonomia. Furthermore, cardiac dysautonomia may occur independent of the presence of a typical restrictive cardiomyopathy. Amyloidosis’ typical restrictive cardiomyopathy is most commonly found in patients with wild-type ATTR type amyloidosis (wtATTR, formerly called senile systemic amyloidosis). In these wtATTR patients, polyneuropathy and dysautonomia are infrequent and approximately 9%.3
At present, actual amyloid infiltration cannot be visualized with nuclear medicine techniques. Nonetheless, semi-quantitative analysis of tracer accumulation in the left ventricle compared to the background (heart-to-mediastinum ratio, HMR) on iodine-123 labelled metaiodobenzylguanidine ([I-123]MIBG) scintigraphy, is assumed to provide insight in the amyloid infiltration of the sympathetic nerve system.4,5,6,7,8,9,10,11,12 [I-123]MIBG, a chemically modified analogue of norepinephrine, is stored in vesicles in presynaptic sympathetic nerve terminals and not further catabolized. Decreased HMR at 4 h after tracer administration (late HMR) reflects the degree of sympathetical dystonia, and is found to be an independent prognostic factor in the development of ventricular dysrhythmia.13 Whereas showing promising results in ischemic heart disease, positron emission tomography (PET) for sympathetic innervation in cardiac manifestation of amyloidosis has not yet been studied.14
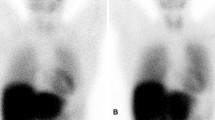
The purpose of this review is to provide an overview of the present literature on the application of nuclear imaging modalities for the evaluation of cardiac innervation in patients with amyloidosis, and its future perspectives (Figures 1, 2, 3).
Methods
For this review a literature search was performed on PubMed on May 5th 2017, using the following string: {[(innervation) OR (sympathetic)] AND [(amyloidosis) OR (amyloid)] AND [(heart) OR (cardiac)] AND [(nuclear) OR (imaging)]}, resulting in 29 hits, of which 24 were considered relevant for this review. Reviews, editorials, abstracts, case reports, animal studies, conference presentations were excluded. In total, 16 articles were found that used radiopharmaceuticals for conventional nuclear medicine imaging of cardiac innervation, all with [I-123]MIBG. The results of these papers are summarized below and divided into three main topics: the imaging of cardiac innervation itself, the implications of this imaging method, and the relation with other nuclear medicine imaging techniques in cardiac amyloidosis.
Imaging of Cardiac Innervation in Amyloidosis
Imaging of cardiac innervation in patients with amyloidosis has been mainly focused on visualizing the effects of amyloidosis on the sympathetic nerve system. Conventional nuclear imaging [I-123]MIBG is the most widely used modality for this indication. Table 1 provides an overview of the present available literature with respect to the use of [I-123]MIBG in patients with different types of systemic amyloidosis. The main results regarding HMR, wash-out and patient outcome of the different studies are displayed. As shown in this overview, hATTR type amyloidosis patients are studied most extensively, showing the most pronounced reduced late HMR. Also AL type amyloidosis patients tend to have decreased late HMR compared to healthy control subjects, however to a lesser extent compared to both hATTR and wtATTR type patients.8,10,12 Due to the large overlap of late HMR ranges in ATTR and AL type amyloidosis patients, [I-123]MIBG scintigraphy is considered not to be able to discriminate between these amyloidosis subtypes.12 Despite that cardiac manifestations are very rare in patients with secondary (AA) amyloidosis, one study showed lower mean late HMR in 11 AA type amyloidosis patients compared to healthy control subjects.12 This finding may contribute to the assumption of amyloid deposits infiltrating the conducting system during the course of the disease.
Mean late HMR differs substantially between the different publications. This variability is mainly due to non-homogeneity in [I-123]MIBG imaging acquisition. HMR varies between different gamma camera systems (venders), but more importantly between the application of low energy and medium energy collimators.15 Generally, HMR is higher on images acquired with medium energy collimators compared to images acquired with low energy collimator.16 Based on these differences in HMR, cut-off values for the different collimators are proposed, as well as conversion algorithms.17,18
Additional single photon emission computed tomography (SPECT) scanning may be of value in the evaluation of regional cardiac sympathetic innervation abnormalities. The majority of patients (both AL and ATTR type amyloidosis) with low HMR show reduced tracer accumulation in the infero-postero-lateral segments.4,5,6,7,8,11 Unfortunately, this may not be considered as a characteristics finding in amyloidosis patients, since a defect in [I-123]MIBG accumulation in the inferior myocardial wall is also reported in healthy control subjects.19 This is considered as a consequence of physiological [I-123]MIBG accumulation in the liver overprojecting the infero-posterior myocardial wall.
Implications of Impaired Cardiac Sympathetic Innervation
Studies using [I-123]MIBG in patients with ischemic heart disease (IHD) have shown that disrupted cardiac sympathetic innervation based on low late HMR is associated with an increased risk on developing ventricular arrhythmia and appropriate implantable cardioverter-defibrillator (ICD) shocks, and is associated with poor survival.13,20 In fact, reduced late HMR is a stronger prognostic factor than left ventricular ejection fraction (LVEF) for developing severe adverse cardiac events in patients with IHD.13 In amyloidosis patients with impaired cardiac sympathetic innervation, decreased survival rates are also established.21,22,23 Late HMR was identified as an independent prognostic factor for 5-year all-cause mortality, with a 42% mortality rate for those patients with late HMR <1.60, compared to merely 7% in patients with late HMR ≥1.60 (hazard ratio (HR) 7.2, P < 0.001).21 Based on the results of this study, even patients with HMR <1.60 seem to benefit from liver transplantation (because of amyloid involvement), resulting in lower long-term mortality than neurophysiological score-matched control subjects (HR 0.32, P = 0.012).21 This underlines the assumption that impaired cardiac sympathetic innervation will not progress after liver transplantation, and that re-innervation cannot be detected within this duration of clinical follow-up.11,23
In addition, late HMR remains of prognostic importance after liver transplantation, with larger area under the receiver-operating curve than clinical parameters and heart rate variability (AUC: 0.79 vs 0.66 and 0.52, respectively) in univariate analysis.22 However, multivariate analysis revealed that late HMR has no additive value to a reference model in predicting outcome (AUC 0.80 vs 0.79, respectively).22
In the AL type population, very little is known about the consequences of reduced late HMR. Follow-up of the available studies in this population is too limited to identify arrhythmogenic consequences of impaired cardiac sympathetic innervation.8,10,12
Data on the contribution of reduced late HMR to cardiovascular outcome measurements in patients with ATTR amyloidosis seems to be incomplete. Only one study reported the association of reduced late HMR with the presence of ventricular arrhythmia, and the progression of conduction disturbances after liver transplantation due to continuous amyloid infiltration.11 Understanding this apparent oxymoron (i.e.: the cessation of progression of cardiac innervation abnormalities despite continuous amyloid infiltration after liver transplantation) will be a challenge for future investigations. As of yet, the actual incidence of ventricular arrhythmia, sudden cardiac death, or appropriate ICD shocks in amyloidosis patients with impaired cardiac sympathetic innervation is not fully elucidated. Therefore, the question whether amyloidosis patients will benefit from prophylactic ICD remains unanswered.
Relation to Other Nuclear Imaging Modalities in Amyloidosis
In early studies using [I-123]MIBG, amyloidosis patients underwent additional (rest) myocardial perfusion scintigraphy using thallium-201 ([Tl-201]).4,5,6,7,9,10,11 None of the included patients seemed to suffer from myocardial infarction, since all rest [Tl-201] scans were reported normal, without perfusion defects. This perfusion – innervation mismatch is a known phenomenon in patients with ischemic cardiomyopathy, but also occurs in patients with non-ischemic (dilating) cardiomyopathy.24,25 Myocardial perfusion abnormalities are known to result in damaged sympathetic nerve terminals, leading to a larger area of impaired innervation than impaired perfusion alone. This mismatch pattern leads to electrophysiological imbalance, which is associated with a higher risk of developing ventricular dysrhythmia.24,25 The mechanism behind the development of perfusion—innervation mismatch pattern in patients with non-ischemic cardiomyopathy is not fully elucidated. However, the presence of structural changes (for example heterogeneous interstitial fibrosis) may contribute to altered ventricular activation and contractility, due to maladaptation to myocardial injury. In combination with disturbed sympathetic stimulation due to amyloid infiltration, this may contribute to a higher risk of ventricular dysrhythmia in amyloidosis patients as well.
The mutual contribution of autonomic neuropathy and cardiomyopathy to each other on decreased late HMR remains a conundrum. Since both wtATTR and hATTR type amyloidosis patients show decreased late HMR, [I-123]MIBG scintigraphy alone may not be sufficient to discriminate between autonomic neuropathy and cardiomyopathy. Several studies have shown that myocardial bone tracer accumulation discriminates ATTR from AL type amyloidosis.26,27 Bone tracer accumulation predominantly occurs in wild-type ATTR type patients, probably as a result of the underlying cardiomyopathy. On the contrary, patients with hATTR type amyloidosis without cardiomyopathy tend to show no myocardial bone tracer accumulation, and normal biomarkers (N-terminus pro-brain natriuretic peptide, and troponine-T). Within these patients, late HMR is generally lower in the subgroup of patients with other symptoms of polyneuropathy.28 Future studies should focus on the possible additive value of bone scintigraphy in relation to [I-123]MIBG scintigraphy in getting a better understanding of the mutual contribution of neuropathy and cardiomyopathy to each other in ATTR type patients.
Recently in positron emission tomography (PET), carbon-11 labelled Pittsburgh compound-B ([C-11]-PiB), derived from the amyloid stain thioflavin, as well as fluorine-18 ([F-18]) labelled florbetapir have been used as tracers for cardiac amyloid.29,30 However, their role against cardiac sympathetic innervation is to be determined. There is no role for [F-18] fluorodeoxyglucose (FDG) imaging or [I-123]SAP scintigraphy in evaluating cardiac manifestation against sympathetic innervation disturbances in amyloidosis, since neither one of both tracers is known to accumulate in cardiac amyloid deposits.31,32
Future Developments
There is an increasing evidence for the prognostic value of [I-123]MIBG scintigraphy in patients with amyloidosis. However, more prospectively acquired data is needed to implement [I-123]MIBG scintigraphy in guidelines as a standard imaging procedure in the management of (especially ATTR type) amyloidosis patients. Therefore, consensus in acquisition parameters in different study protocols is pivotal. Standardization of collimator choice, imaging acquisition, and data analysis in different studies, is necessary for successful implementation in daily patient practice.15
Finally, the use of PET tracers has advantages over [I-123]MIBG in cardiac sympathetic innervation imaging. Carbon-11 labelled meta-hydroxy-ephedrine [C-11]mHED has been extensively studied in patients with both ischemic and non-ischemic cardiomyopathies.14,20 Based on the studies in patients with left ventricular dysfunction, [C-11]mHED outperforms [I-123]MIBG in detecting regional impaired sympathetic innervation, due to better resolution and absolute quantification.33 Despite that [C-11]mHED is the most used PET tracer for visualization of cardiac sympathetic innervation abnormalities, it’s value has not yet been studied in amyloidosis patients. Future studies should provide information on the value of recently developed PET tracers in evaluating cardiac sympathetic innervation in amyloidosis patients. In theory, two new PET tracers may have additional value over [I-123]MIBG scintigraphy in regard to higher HMR. For example, [I-124]MIBG may provide superior image quality, whereas N-[3-Bromo-4-3-[F-18]fluoro-propoxy)-benzyl]-guanidine ([F-18]LM1195) has the additional advantage that an on-site cyclotron is not necessary.34
Conclusions
[I-123]MIBG is currently the most widely used radiopharmaceutical for imaging cardiac sympathetic innervation disturbances in patients with cardiac manifestations of amyloidosis. Particular patients with hATTR type amyloidosis show diminished late HMR’s, and consequently have a higher risk of cardiac mortality.
Future studies should provide better insight into the presence and degree of overlap between cardiac neuropathy and cardiomyopathy in patients with cardiac manifestations of amyloidosis, the role of nuclear medicine modalities in distinguishing cardiac neuropathy from cardiomyopathy, and finally, the potential role of PET tracers in evaluating impaired cardiac sympathetic innervation.
References
Goldstein DS. Cardiac dysautonomia and survival in hereditary transthyretin amyloidosis. JACC Cardiovasc Imaging. 2016;12:1442-5.
Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 1997;337:898-900.
Pinney JH, Whelan CJ, Petrie A, Dungu J, Banypersad SM, Sattianayagam P, et al. Senile systemic amyloidosis: Clinical features at presentation and outcome. J Am Heart Assoc. 2013;2:e000098.
Nakata T, Shimamoto K, Yonekura S, Kobayashi N, Sugiyama T, Imai K, et al. Cardiac sympathetic denervation in transthyretin-related familial amyloidotic polyneuropathy: Detection with iodine-123-MIBG. J Nucl Med. 1995;36:1040-2.
Tanaka M, Hongo M, Kinoshita O, Takabayashi Y, Fujii T, Yazaki Y, et al. Iodine-123 metaiodobenzylguanidine scintigraphic assessment of myocardial sympathetic innervation in patients with familial amyloid polyneuropathy. J Am Coll Cardiol. 1997;29:168-74.
Delahaye N, Dinanian S, Slama MS, Mzabi H, Samuel D, Adams D, et al. Cardiac sympathetic denervation in familial amyloid polyneuropathy assessed by iodine-123 metaiodobenzylguanidine scintigraphy and heart rate variability. Eur J Nucl Med. 1999;26:416-24.
Arbab AS, Koizumi K, Toyama K, Arai T, Yoshitomi T, Araki T. Scan findings of various myocardial SPECT agents in a case of amyloid polyneuropathy with suspected myocardial involvement. Ann Nucl Med. 1997;11:139-41.
Hongo M, Urushibata K, Kai R, Takahashi W, Koizumi T, Uchikawa S, et al. Iodine-123 metaiodobenzylguanidine scintigraphic analysis of myocardial sympathetic innervation in patients with AL (primary) amyloidosis. Am Heart J. 2002;144:122-9.
Watanabe H, Misu K, Hirayama M, Hattori N, Yoshihara T, Doyu M, et al. Low cardiac 123I-MIBG uptake in late-onset familial amyloid polyneuropathy type I (TTR Met30). J Neurol. 2001;248:627-9.
Lekakis J, Dimopoulos MA, Prassopoulos V, Mavrikakis M, Gerali S, Sifakis N, et al. Myocardial adrenergic denervation in patients with primary (AL) amyloidosis. Amyloid. 2003;10:117-20.
Delahaye N, Rouzet F, Sarda L, Tamas C, Dinanian S, Plante-Bordeneuve V, et al. Impact of liver transplantation on cardiac autonomic denervation in familial amyloid polyneuropathy. Medicine (Baltimore). 2006;85:229-38.
Noordzij W, Glaudemans AW, van Rheenen RW, Hazenberg BP, Tio RA, Dierckx RA, et al. (123)I-Labelled metaiodobenzylguanidine for the evaluation of cardiac sympathetic denervation in early stage amyloidosis. Eur J Nucl Med Mol Imaging. 2012;39:1609-17.
Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol. 2010;55:2212-21.
Fallavollita JA, Heavey BM, Luisi AJ Jr, Michalek SM, Baldwa S, Mashtare TL Jr, et al. Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J Am Coll Cardiol. 2014;63:141-9.
Flotats A, Carrió I, Agostini D, Le Guludec D, Marcassa C, Schäfers M, et al. Proposal for standardization of 123I-metaiodobenzylguanidine (MIBG) cardiac sympathetic imaging by the EANM Cardiovascular Committee and the European Council of Nuclear Cardiology. Eur J Nucl Med Mol Imaging. 2010;37:1802-12.
Nakajima K, Matsumoto N, Kasai T, Matsuo S, Kiso K, Okuda K. Normal values and standardization of parameters in nuclear cardiology: Japanese Society of Nuclear Medicine working group database. Ann Nucl Med. 2016;30:188-99.
Inoue Y, Abe Y, Kikuchi K, Matsunaga K, Masuda R, Nishiyama K. Correction of collimator-dependent differences in the heart-to-mediastinum ratio in 123I-metaiodobenzylguanidine cardiac sympathetic imaging: Determination of conversion equations using point-source imaging. J Nucl Cardiol 2016;1-12.
Nakajima K, Verschure DO, Okuda K, Verberne HJ. Standardization of 123I-meta-iodobenzylguanidine myocardial sympathetic activity imaging: Phantom calibration and clinical applications. Clin Transl Imaging. 2017;5:255-63.
Gill JS, Hunter GJ, Gane G, Camm AJ. Heterogeneity of the human myocardial sympathetic innervation: In vivo demonstration by iodine 123-labeled meta-iodobenzylguanidine scintigraphy. Am Heart J. 1993;126:390-8.
Pietilä M, Malminiemi K, Ukkonen H, Saraste M, Någren K, Lehikoinen P, et al. Reduced myocardial carbon-11 hydroxyephedrine retention is associated with poor prognosis in chronic heart failure. Eur J Nucl Med. 2001;28:373-6.
Coutinho MC, Cortez-Dias N, Cantinho G, Conceição I, Oliveira A, Bordalo e Sá A, et al. Reduced myocardial 123-iodine metaiodobenzylguanidine uptake: A prognostic marker in familial amyloid polyneuropathy. Circ Cardiovasc Imaging. 2013;6:627-36.
Algalarrondo V, Antonini T, Théaudin M, Chemla D, Benmalek A, Lacroix C, et al. Cardiac dysautonomia predicts long-term survival in hereditary transthyretin amyloidosis after liver transplantation. JACC Cardiovasc Imaging. 2016;9:1432-41.
Azevedo Coutinho MDC, Cortez-Dias N, Cantinho G, Conceição I, Guimarães T, Lima da Silva G, et al. Progression of myocardial sympathetic denervation assessed by 123I-MIBG imaging in familial amyloid polyneuropathy and the effect of liver transplantation. Rev Port Cardiol. 2017;36:333-40.
Simões MV, Barthel P, Matsunari I, Nekolla SG, Schömig A, Schwaiger M, et al. Presence of sympathetically denervated but viable myocardium and its electrophysiologic correlates after early revascularised, acute myocardial infarction. Eur Heart J. 2004;25:551-7.
Sasano T, Abraham R, Chang KC, Ashikaga H, Mills KJ, Holt DP, et al. Abnormal sympathetic innervation of viable myocardium and the substrate of ventricular tachycardia after myocardial infarction. J Am Coll Cardiol. 2008;51:2266-75.
Perugini E, Guidalotti PL, Salvi F, Cooke RM, Pettinato C, Riva L, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. 2005;46:1076-84.
Bokhari S, Castaño A, Pozniakoff T, Deslisle S, Latif F. Maurer MS () (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging. 2013;6:195-201.
Coutinho CA, Conceição I, Almeida A, Cantinho G, Sargento L, Vagueiro MC. Early detection of sympathetic myocardial denervation in patients with familial amyloid polyneuropathy type I. Rev Port Cardiol. 2004;23:201-11.
Antoni G, Lubberink M, Estrada S, Axelsson J, Carlson K, Lindsjo L, et al. In vivo visualization of amyloid deposits in the heart with 11C-PIB and PET. J Nucl Med. 2013;54:213-20.
Dorbala S, Vangala D, Semer J, Strader C, Bruyere JR Jr, Di Carli MF, et al. Imaging cardiac amyloidosis: A pilot study using 18F-florbetapir positron emission tomography. Eur J Nucl Med Mol Imaging. 2014;41:1652-62.
Mekinian A, Jaccard A, Soussan M, Launay D, Berthier S, Federici L, et al. 18F-FDG PET/CT in patients with amyloid light-chain amyloidosis: Case-series and literature review. Amyloid. 2012;19:94-8.
Hazenberg BP, van Rijswijk MH, Lub-de Hooge MN, Vellenga E, Haagsma EB, Posthumus MD, et al. Diagnostic performance and prognostic value of extravascular retention of 123I-labeled serum amyloid P component in systemic amyloidosis. J Nucl Med. 2007;48:865-72.
Matsunari I, Aoki H, Nomura Y, Takeda N, Chen WP, Taki J, et al. Iodine-123 metaiodobenzylguanidine imaging and carbon-11 hydroxyephedrine positron emission tomography compared in patients with left ventricular dysfunction. Circ Cardiovasc Imaging. 2010;3:595-603.
Werner RA, Rischpler C, Onthank D, Lapa C, Robinson S, Samnick S, et al. Retention kinetics of the 18F-labeled sympathetic nerve PET tracer LMI1195: Comparison with 11C-hydroxyephedrine and 123I-MIBG. J Nucl Med. 2015;56:1429-33.
Delahaye N, Le Guludec D, Dinanian S, Delforge J, Slama MS, Sarda L, et al. Myocardial muscarinic receptor upregulation and normal response to isoproterenol in denervated hearts by familial amyloid polyneuropathy. Circulation. 2001;104:2911-6.
Algalarrondo V, Eliahou L, Thierry I, Bouzeman A, Dasoveanu M, Sebag C, et al. Circadian rhythm of blood pressure reflects the severity of cardiac impairment in familial amyloid polyneuropathy. Arch Cardiovasc Dis. 2012;105:281-90.
Noordzij W, Glaudemans AW, Slart RH, Dierckx RA, Hazenberg BP. Clinical use of differential nuclear medicine modalities in patients with ATTR amyloidosis. Amyloid. 2012;19:208-11.
Takahashi R, Ono K, Shibata S, Nakamura K, Komatsu J, Ikeda Y, et al. Efficacy of diflunisal on autonomic dysfunction of late-onset familial amyloid polyneuropathy (TTR Val30Met) in a Japanese endemic area. J Neurol Sci. 2014;345:231-5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
All Authors have no disclosure to state.
Additional information
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarizes the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Slart, R.H.J.A., Glaudemans, A.W.J.M., Hazenberg, B.P.C. et al. Imaging cardiac innervation in amyloidosis. J. Nucl. Cardiol. 26, 174–187 (2019). https://doi.org/10.1007/s12350-017-1059-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-017-1059-9