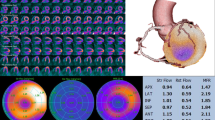
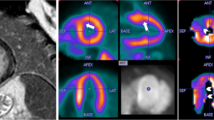
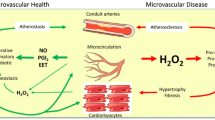
Abstract
Infiltrative heart diseases are characterized by myocardial tissue alterations leading to mechanical dysfunction which in turn develops into bi-ventricular congestive heart failure. Also the coronary microvasculature undergoes significant remodeling and dysfunction. The effects of the unbalance of the mechanical cross-talk between cardiac muscle and vessels and of the impairment of vasodilatory function can be measured non-invasively by means of positron emission tomography and cardiac magnetic resonance.



Similar content being viewed by others
References
Rapezzi C, Merlini G, Quarta CC, Riva L, Longhi S, Leone O, et al. Systemic cardiac amyloidoses: Disease profiles and clinical courses of the 3 main types. Circulation 2009;120:1203-12.
Maleszewski JJ. Cardiac amyloidosis: Pathology, nomenclature, and typing. Cardiovasc Pathol 2015;24:343-50.
Larsen BT, Mereuta OM, Dasari S, Fayyaz AU, Theis JD, Vrana JA, et al. Correlation of histomorphological pattern of cardiac amyloid deposition with amyloid type: A histological and proteomic analysis of 108 cases. Histopathology 2016;68:648-56.
Camici PG, d’Amati G, Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol 2015;12:48-62.
Toyota E, Koshida R, Hattan N, Chilian WM. Regulation of the coronary vasomotor tone: What we know and where we need to go. J Nucl Cardiol 2001;8:599-605.
Pries AR, Badimon L, Bugiardini R, Camici PG, Dorobantu M, Duncker DJ, et al. Coronary vascular regulation, remodelling, and collateralization: Mechanisms and clinical implications on behalf of the working group on coronary pathophysiology and microcirculation. Eur Heart J 2015;36:3134-46.
Tune JD. Coronary circulation. In: Granger ND, Granger J, editors. Colloquium series on integrated systems physiology: From molecule to function. New York: Springer; 2014. p. 1-89.
Crea F, Lanza GA, Camici PG. Coronary microvascular dysfunction. Milan: Springer; 2014.
Whitaker DC, Tungekar MF, Dussek JE. Angina with a normal coronary angiogram caused by amyloidosis. Heart 2004;90:e54.
Dorbala S, Vangala D, Bruyere J Jr, Quarta C, Kruger J, Padera R, et al. Coronary microvascular dysfunction is related to abnormalities in myocardial structure and function in cardiac amyloidosis. JACC Heart Fail 2014;2:358-67.
Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007;356:830-40.
Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J 2014;35:1101-11.
Teunissen PFA, de Waard GA, Hollander MR, Robbers LFHJ, Danad I, Biesbroek PS, et al. Doppler-derived intracoronary physiology indices predict the occurrence of microvascular injury and microvascular perfusion deficits after angiographically successful primary percutaneous coronary intervention. Circulation 2015;8:e001786.
van de Hoef TP, Nolte F, Rolandi MC, Piek JJ, van den Wijngaard JP, Spaan JA, et al. Coronary pressure-flow relations as basis for the understanding of coronary physiology. J Mol Cell Cardiol 2012;52:786-93.
Bravo PE, Di Carli MF, Dorbala S. Role of PET to evaluate coronary microvascular dysfunction in non-ischemic cardiomyopathies. Heart Fail Rev 2017;22:455-64.
Heydari B, Kwong RY, Jerosch-Herold M. Technical advances and clinical applications of quantitative myocardial blood flow imaging with cardiac MRI. Prog Cardiovasc Dis 2015;57:615-22.
Hautvast GL, Chiribiri A, Lockie T, Breeuwer M, Nagel E, Plein S. Quantitative analysis of transmural gradients in myocardial perfusion magnetic resonance images. Magn Reson Med 2011;66:1477-87.
Sipe JD, Benson MD, Buxbaum JN, Ikeda S, Merlini G, Saraiva MJ, et al. Nomenclature 2014: Amyloid fibril proteins and clinical classification of the amyloidosis. Amyloid 2014;21:221-24.
Crotty TB, Li CY, Edwards WD, Suman VJ. Amyloidosis and endomyocardial biopsy: Correlation of extent and pattern of deposition with amyloid immunophenotype in 100 cases. Cardiovasc Pathol 1995;4:39-42.
Biolo A, Ramamurthy S, Connors LH, O’Hara CJ, Meier-Ewert HK, Soo Hoo PT, et al. Matrix metalloproteinases and their tissue inhibitors in cardiac amyloidosis: Relationship to structural, functional myocardial changes and to light chain amyloid deposition. Circ Heart Fail 2008;1:249-57.
McWilliams-Koeppen HP, Foster JS, Hackenbrack N, Ramirez-Alvarado M, Donohoe D, Williams A, et al. Light chain amyloid fibrils cause metabolic dysfunction in human cardiomyocytes. PLoS ONE 2015;10:e0137716.
Fontana M, Banypersad SM, Treibel TA, Abdel-Gadir A, Maestrini V, Lane T, et al. Differential myocyte responses in patients with cardiac transthyretin amyloidosis and light-chain amyloidosis: A cardiac MR imaging study. Radiology 2015;277:388-97.
Ong KC, Askew JW, Dispenzieri A, Maleszewski JJ, Klarich KW, Anavekar NS, et al. Abnormal stress echocardiography findings in cardiac amyloidosis. Amyloid 2016;23:124-31.
Falk RH, Alexander KM, Liao R, Dorbala S. AL (light-chain) cardiac amyloidosis: A review of diagnosis and therapy. J Am Coll Cardiol 2016;68:1323-41.
Wittich CM, Neben-Wittich MA, Mueller PS, Gertz MA, Edwards WD. Deposition of amyloid proteins in the epicardial coronary arteries of 58 patients with primary systemic amyloidosis. Cardiovasc Pathol 2007;16:75-78.
Smith RR, Hutchins GM. Ischemic heart disease secondary to amyloidosis of intramyocardial arteries. Am J Cardiol 1979;44:413-7.
Neben-Wittich MA, Wittich CM, Mueller PS, Larson DR, Gertz MA, Edwards WD. Obstructive intramural coronary amyloidosis and myocardial ischemia are common in primary amyloidosis. Am J Med 2005;118:1287.
Brenner DA, Jain M, Pimentel DR, Wang B, Connors LH, Skinner M, et al. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ Res 2004;94:1008-10.
Migrino RQ, Truran S, Gutterman DD, Franco DA, Bright M, Schlundt B, et al. Human microvascular dysfunction and apoptotic injury induced by AL amyloidosis light chain proteins. Am J Physiol Heart Circ Physiol 2011;301:H2305-12.
Coutinho MCA, Cortez-Dias N, Cantinho G, Conceição I, Oliveira A, Bordalo e Sá A, et al. Reduced myocardial 123-iodine metaiodobenzylguanidine uptake a prognostic marker in familial amyloid polyneuropathy. Circ Cardiovasc Imaging 2013;6:627-36.
Noordzij W, Glaudemans AW, van Rheenen RW, Hazenberg BP, Tio RA, Dierckx RA, et al. (123)I-Labelled metaiodobenzylguanidine for the evaluation of cardiac sympathetic denervation in early stage amyloidosis. Eur J Nucl Med Mol Imaging 2012;39:1609-17.
Al Suwaidi J, Velianou JL, Gertz MA, Cannon RO 3rd, Higano ST, Holmes DR Jr, et al. Systemic amyloidosis presenting with angina pectoris. Ann Intern Med 1999;131:838-41.
Dorbala S, Vangala D, Semer J, Strader C, Bruyere JR Jr, Di Carli MF, et al. Imaging cardiac amyloidosis: A pilot study using (1)(8)F-florbetapir positron emission tomography. Eur J Nucl Med Mol Imaging 2014;41:1652-62.
Bokhari S, Shahzad R, Castaño A, Maurer MS. Nuclear imaging modalities for cardiac amyloidosis. J Nucl Cardiol 2014;21:175-84.
Camici PG, Rimoldi OE. The clinical value of myocardial blood flow measurement. J Nucl Med 2009;50:1076-87.
Fontana M, Banypersad SM, Treibel TA, Maestrini V, Sado DM, White SK, et al. Native T1 mapping in transthyretin amyloidosis. JACC Cardiovasc Imaging 2014;7:157-65.
Li R, Yang ZG, Wen LY, Liu X, Xu HY, Zhang Q, et al. Regional myocardial microvascular dysfunction in cardiac amyloid light-chain amyloidosis: Assessment with 3T cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2016;18:16.
Desnick RJ, Wasserstein MP. Fabry disease: Clinical features and recent advances in enzyme replacement therapy. Adv Nephrol Necker Hosp 2001;31:317-39.
Germain DP. Fabry disease. Orphanet J Rare Dis 2010;5:30.
Nakao S, Takenaka T, Maeda M, Kodama C, Tanaka A, Tahara M, et al. An atypical variant of Fabry’s disease in men with left ventricular hypertrophy. N Engl J Med 1995;333:288-93.
Shah JS, Lee P, Hughes D, Thaman R, Sachdev B, Pellerin D, et al. The natural history of left ventricular systolic function in Anderson-Fabry disease. Heart 2005;91:533-34.
O’Mahony C, Elliott P. Anderson-Fabry disease and the heart. Prog Cardiovasc Dis 2010;52:326-35.
Chimenti C, Morgante E, Tanzilli G, Mangieri E, Critelli G, Gaudio C, et al. Angina in fabry disease reflects coronary small vessel disease. Circ Heart Fail 2008;1:161-69.
Elliott PM, Kindler H, Shah JS, Sachdev B, Rimoldi OE, Thaman R, et al. Coronary microvascular dysfunction in male patients with Anderson-Fabry disease and the effect of treatment with alpha galactosidase A. Heart 2006;92:357-60.
Kalliokoski RJ, Kalliokoski KK, Sundell J, Engblom E, Penttinen M, Kantola I, et al. Impaired myocardial perfusion reserve but preserved peripheral endothelial function in patients with Fabry disease. J Inherit Metab Dis 2005;28:563-73.
Tomberli B, Cecchi F, Sciagra R, Berti V, Lisi F, Torricelli F, et al. Coronary microvascular dysfunction is an early feature of cardiac involvement in patients with Anderson-Fabry disease. Eur J Heart Fail 2013;15:1363-73.
Lynch JP 3rd, Hwang J, Bradfield J, Fishbein M, Shivkumar K, Tung R. Cardiac involvement in sarcoidosis: Evolving concepts in diagnosis and treatment. Semin Respir Crit Care Med 2014;35:372-90.
Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 2014;63:329-36.
Vaccarino V, Khan D, Votaw J, Faber T, Veledar E, Jones DP, et al. Inflammation is related to coronary flow reserve detected by positron emission tomography in asymptomatic male twins. J Am Coll Cardiol 2011;57:1271-79.
Recio-Mayoral A, Rimoldi OE, Camici PG, Kaski JC. Inflammation and microvascular dysfunction in cardiac syndrome X patients without conventional risk factors for coronary artery disease. JACC Cardiovasc Imaging 2013;6:660-67.
Recio-Mayoral A, Mason JC, Kaski JC, Rubens MB, Harari OA, Camici PG. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J 2009;30:1837-43.
Kruse MJ, Kovell L, Kasper EK, Pomper MG, Moller DR, Solnes L, et al. Myocardial Blood flow and inflammatory cardiac sarcoidosis. JACC Cardiovasc Imaging 2017;10:157-67.
Dweck MR, Abgral R, Trivieri MG, Robson PM, Karakatsanis N, Mani V, et al. Hybrid magnetic resonance imaging and positron emission tomography with fluorodeoxyglucose to diagnose active cardiac sarcoidosis. JACC Cardiovasc Imaging 2017. doi:10.1016/j.jcmg.2017.02.021.
Disclosure
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter discussed in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rimoldi, O., Maranta, F. Microvascular dysfunction in infiltrative cardiomyopathies. J. Nucl. Cardiol. 26, 200–207 (2019). https://doi.org/10.1007/s12350-017-0991-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-017-0991-z