Abstract
Introduction
Accurate assessment of estrogen receptor (ER) expression is crucial to ensure that patients with early breast cancer are accurately identified for appropriate treatment with endocrine therapy. Reverse transcriptase polymerase chain reaction (RT-PCR), compared with immunohistochemistry (IHC), may provide a more precise indication of ER status. Data were pooled and analyzed from two independent, but similarly designed, studies that examined ER status by IHC and the 21-gene Recurrence Score that employs RT-PCR-based methodology.
Methods
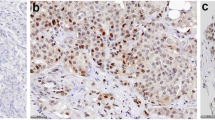
Tumor tissue from patients with early stage breast cancer where ER status could be determined by both IHC and RT-PCR was included. ER status by IHC staining was defined as ER-negative (< 1%), ER-low+ (1–10%), or ER+ (> 10%). ER status by RT-PCR was defined as ER-negative (≤ 6.5) or ER+ (> 6.5). Recurrence Score results from the 21-gene assay were reported on a continuous scale from 0 to 100. A sub-analysis examined the association between ER expression (Allred score 2–7) and response to a 14-day pre-surgery pulse with an aromatase inhibitor. A separate sub-analysis examined the association between ER expression and human epidermal growth factor receptor 2 (HER2) expression.
Results
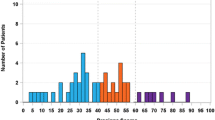
Tumor specimens from 192 patients (aged 25–92 years) were included in the pooled analysis. Correlation between IHC- and RT-PCR-measured ER was strong for IHC-defined ER-negative and ER+ samples (r = 0.646 [95% CI 0.553–0.720]). There was 100% concordance for ER+ tumors; however, 56% of the ER-low+ tumors were negative by RT-PCR. Allred score correlated better with ER status measured by RT-PCR at pre-treatment (r = 0.83) than at post-treatment (r = 0.76). The majority (77%) of ER-negative and ER-low+ tumors were HER2-negative.
Conclusions
RT-PCR provided a more accurate assessment of ER expression in patients with ER-low+ tumors, and data support dual testing for patients with ER-low+ status to ensure appropriate treatment planning as it pertains to endocrine therapy.
Funding
Genomic Health, Inc.





Similar content being viewed by others
References
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–84.
National Comprehensive Cancer Network (NCCN) practice guidelines in oncology. Breast cancer. www.nccn.org, Version 2.2018. Accessed 15 Oct 2018.
Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2014;32:2255–69.
Dowsett M, Allred C, Knox J, et al. Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or in Combination trial. J Clin Oncol. 2008;26:1059–65.
Hammond MEH, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–95.
Dowsett M, Sestak I, Buus R, et al. Estrogen receptor expression in 21-gene recurrence score predicts increased late recurrence for estrogen-positive/HER2-negative breast cancer. Clin Cancer Res. 2015;21:2763–70.
DeSantis C, Howlader N, Cronin KA, Jemal A. Breast cancer incidence rates in U.S. women are no longer declining. Cancer Epidemiol Biomarkers Prev. 2011;20:733–9.
Lim E, Metzger-Filho O, Winer EP. The natural history of hormone receptor-positive breast cancer. Oncology. 2012;26:688.
Prabhu JS, Korlimarla A, Desai K, et al. A majority of low (1–10%) ER positive breast cancers behave like hormone receptor negative tumors. J Cancer. 2014;5:156–65.
Yi M, Huo L, Koenig KB, et al. Which threshold for ER positivity? A retrospective study based on 9639 patients. Ann Oncol. 2014;25:1004–111.
Deyarmin B, Kane JL, Valente AL, et al. Effect of ASCO/CAP guidelines for determining ER status on molecular subtype. Ann Surg Oncol. 2013;1:87–93.
American Cancer Society. Targeted therapy for breast cancer. https://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-treating-targeted-therapy. 2018. Accessed 30 Oct 2018.
Bentzon N, Düring M, Rasmussen BB, Mouridsen H, Kroman N. Prognostic effect of estrogen receptor status across age in primary breast cancer. Int J Cancer. 2008;122:1089–94.
Yu KD, Wu J, Shen ZZ, Shao ZM. Hazard of breast cancer-specific mortality among women with estrogen receptor-positive breast cancer after five years from diagnosis: implication for extended endocrine therapy. J Clin Endocrinol Metabol. 2012;97:E2201–9.
Verma S, Provencher L, Dent R. Emerging trends in the treatment of triple-negative breast cancer in Canada: a survey. Current Oncology. 2011;18:180–90.
Iwamoto T, Booser D, Valero V, et al. Estrogen receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1% to 10% ER-positive by immunohistochemistry. J Clin Oncol. 2012;30:729–34.
Badve SS, Baehner FL, Gray R, et al. Estrogen- and progesterone-receptor status in ECOG 2197: comparison of immunohistochemistry by local and central laboratories and quantitative reverse transcription polymerase chain reaction by central laboratory. J Clin Oncol. 2008;26:2473–81.
Baehner FL, Maddala T, Alexander C, et al. A Kaiser Permanente population-based study of ER and PR expression by the standard method, immunohistochemistry (IHC), compared to a new method, quantitative reverse transcription polymerase chain reaction (RT-PCR). In: Presented at the ASCO Breast Cancer Symposium. September 7-8, 2007; San Francisco, CA. Abstract #88.
O'Connor SM, Beriwal S, Dabbs DJ, Bhargava R. Concordance between semiquantitative immunohistochemical assay and oncotype DX RT-PCR assay for estrogen and progesterone receptors. Appl Immunohistochem Mol Morphol. 2010;18:268–72.
Kim C, Tang G, Pogue-Geile KL, et al. Estrogen receptor (ESR1) mRNA expression and benefit from tamoxifen in the treatment and prevention of estrogen receptor-positive breast cancer. J Clin Oncol. 2011;29:4160–7.
Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–26.
Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–34.
Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene Recurrence Score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a transATAC study. J Clin Oncol. 2010;28:1829–34.
Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65.
Discordance in hormone receptor (HR) assessment by IHC and RT-PCR in an estrogen receptor (ER) low-positive group (1–10% positive cells): Does accurate assessment of HR status require dual testing? Presented at the United States and Canadian Academy of Pathology (USCAP) Annual Meeting, March 2014; San Diego, CA.
Baehner FL, Achacoso N, Maddala T, et al. Human epidermal growth factor receptor 2 assessment in a case-control study: comparison of fluorescence in situ hybridization and quantitative reverse transcription performed by central laboratories. J Clin Oncol. 2010;28:4300–6.
Dixon JM, Turnbull A, Renshaw L, et al. Association of estrogen receptor (ER) levels and prediction of antiproliferative effect of hormone therapy (HT) in lower ER-expressing tumors. Presented at the San Antonio Breast Cancer Symposium (SABCS) Annual Meeting; December 2014; San Antonio, TX.
Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Modern Pathol. 1998;11:155–68.
Inwald EC, Klinkhammer-Schalke M, Hofstädter F, et al. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat. 2013;139:539–52.
Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43.
Allred DC, Carlson RW, Berry DA, et al. NCCN Task Force report: estrogen receptor and progesterone receptor testing in breast cancer by immunohistochemistry. J Natl Compr Cancer Netw. 2009;7(suppl 6):S1–21.
Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379:111–21.
Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol. 2005;23:7212–20.
Dowsett M, Nielsen TO, A’Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103:1–9.
Akashi-Tanaka S, Shimizu C, Ando M, et al. 21-gene expression profile assay on core needle biopsies predicts responses to neoadjuvant endocrine therapy in breast cancer patients. Breast. 2009;18:171–4.
Iwata H, Masuda N, Yamamoto Y, et al. Validation of the 21-gene test as a predictor of clinical response to neoadjuvant hormonal therapy for ER+, HER2-negative breast cancer: the TransNEOS study. Breast Cancer Res Treat. 2018. https://doi.org/10.1007/s10549-018-4964-y.
Ueno T, Masuda N, Yamanaka T, et al. Evaluating the 21-gene assay Recurrence Score® as a predictor of clinical response to 24 weeks of neoadjuvant exemestane in estrogen receptor-positive breast cancer. Int J Clin Oncol. 2014;19:607–13.
Müller BM, Kronenwett R, Hennig G, et al. Quantitative determination of estrogen receptor, progesterone receptor, and HER2 mRNA in formalin-fixed paraffin-embedded tissue—a new option for predictive biomarker assessment in breast cancer. Diagn Mol Pathol. 2011;20:1–10.
Acknowledgements
J. Michael Dixon and Arran Turnbull acknowledge the support of Breast Cancer Now. The authors would like to sincerely thank Amy Sing, MD (formerly an employee of Genomic Health, Inc.) for her coordination of the study and significant intellectual contributions to the development of the initial draft of manuscript.
Funding
This analysis was funded by Genomic Health. The study sponsor, Genomic Health, Inc., also funded the article processing charges for this article. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and Editorial Assistance
Writing, editorial, and graphics support for the development of this manuscript was provided by Penny Baron, MS and Robert Steger, PhD of CodonMedical, an Ashfield Company, part of UDG Healthcare plc, funded by Genomic Health, Redwood City, CA.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Prior Presentation
Information in this article has been presented previously, in part, as posters:
-
Sing A, Dixon JM, Turnbull A, et al. Association of estrogen receptor (ER) levels and prediction of antiproliferative effect of hormone therapy in lower ER-expressing tumors. 37th Annual San Antonio Breast Cancer Symposium (SABCS 2014); December 9–13, 2013; San Antonio, Texas. Abstract #P3-06-35.
-
Singh B, Ziguridis N, Axelrod D, et al. Discordance in hormone receptor (HR) assessment by IHC and RT-PCR in an estrogen receptor (ER) low-positive group (1–10% positive cells): Does accurate assessment of HR status require dual testing? 36th Annual San Antonio Breast Cancer Symposium (SABCS 2013); December 10–14, 2013; San Antonio, Texas. Abstract #P3-05-08.
-
Singh B, Ziguridis N, Butler S, et al. Comparison of HER2 testing by IHC/FISH and RT-PCR in estrogen receptor negative or borderline patients with early stage breast cancer. 102nd United States and Canadian Academy of Pathology (USCAP) Annual Meeting; March 2–8, 2013; Baltimore, Maryland. Abstract #282.
Disclosures
J. Michael Dixon, Laura M. Arthur, Deborah M. Axelrod, Lorna Renshaw, Arran Turnbull, and Oliver Young declare that they have no conflict of interest. Jeremy S. Thomas has moved affiliations and is now employed by Q2 Solutions, The Alba Campus, Rosebank, Livingston, UK. He has no conflicts of interest to declare. David A. Cameron attended an advisory board for Genomic Health, Inc. (any income associated with the advisory board was paid to Dr. Cameron’s employer and none to Dr. Cameron). Cynthia A. Loman is an employee of and declares stock ownership in Genomic Health, Inc. Debbie Jakubowski is an employee of and declares stock ownership in Genomic Health, Inc. Frederick L. Baehner is an employee of and declares stock ownership in Genomic Health, Inc. Baljit Singh is a member of the speakers’ bureau for Genomic Health, Inc. and was previously employed (during the conduct of Study 1) by New York University Langone Medical Center, New York, NY, USA.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human or animal subjects performed by any of the authors. In study 1 (New York University), the link between patient identifiers and clinico/pathological data was permanently deleted and the study was 'exempt' from review by the New York University Internal Review Board. Patients in study 2 (Edinburgh Breast Unit) were recruited under ethical approval from the South East Scotland Research Ethics Committee 03, approved in 2002, reference LREC/2002/8/23.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7649864.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dixon, J.M., Cameron, D.A., Arthur, L.M. et al. Accurate Estrogen Receptor Quantification in Patients with Negative and Low-Positive Estrogen-Receptor-Expressing Breast Tumors: Sub-Analyses of Data from Two Clinical Studies. Adv Ther 36, 828–841 (2019). https://doi.org/10.1007/s12325-019-0896-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-0896-0