Abstract
Introduction
Clostridium difficile infection (CDI) is the major cause of infectious nosocomial diarrhoea and is associated with considerable morbidity, mortality and economic impact. Bezlotoxumab administered in combination with standard of care (SoC) antibiotic therapy prevents recurrent CDI. This study assessed the cost-effectiveness of bezlotoxumab added to SoC, compared to SoC alone, to prevent the recurrence of CDI in high-risk patients from the Spanish National Health System perspective.
Methods
A Markov model was used to simulate the natural history of CDI over a lifetime horizon in five populations of patients at high risk of CDI recurrence according to MODIFY trials: (1) ≥ 65 years old; (2) severe CDI; (3) immunocompromised; (4) ≥ 1 CDI episode in the previous 6 months; and (5) ≥ 65 years old and with ≥ 1 CDI episode in the previous 6 months. The incremental cost-effectiveness ratio (ICER) expressed as cost per quality-adjusted life-year (QALY) gained was calculated. Deterministic (DSA) and probabilistic sensitivity analyses (PSA) were performed.
Results
In all patient populations (from 1 to 5), bezlotoxumab added to SoC reduced CDI recurrence compared to SoC alone by 26.4, 19.5, 21.2, 26.6 and 39.7%, respectively. The resulting ICERs for the respective subgroups were €12,724, €17,495, €9545, €7386, and €4378. The model parameters with highest impact on the ICER were recurrence rate (first), mortality, and utility values. The probability that bezlotoxumab was cost-effective at a willingness-to-pay threshold of €21,000/QALY was 85.5%, 54.1%, 86.0%, 94.5%, 99.6%, respectively.
Conclusion
The results suggest that bezlotoxumab added to SoC compared to SoC alone is a cost-effective treatment to prevent the recurrence of CDI in high-risk patients. The influence of changes in model parameters on DSA results was higher in patients ≥ 65 years old, with severe CDI and immunocompromised. Additionally, PSA estimated that the probability of cost-effectiveness exceeded 85% in most subgroups.
Funding
Merck Sharp & Dohme Corp.
Similar content being viewed by others
Introduction
Clostridium difficile infection (CDI) is the major cause of infectious nosocomial diarrhoea and is associated with considerable morbidity and mortality, as well as a significant economic impact on the healthcare systems of developed countries [1]. The incidence of CDI increased by 70% in European countries from 4.1 to 7.0 cases per 10,000 patient-bed days between 2008 and 2013, without taking into account the existence of a considerable number of potentially missed CDI diagnoses due to the frequent use of suboptimum laboratory diagnostic tests [2]. In Spain, the estimated figure was 3.2 cases per 10,000 patient-bed days in 2013 [2], with half of the episodes underdiagnosed [3].
The clinical burden of CDI ranges from symptomless carriage, through mild or moderate diarrhoea, to fulminant and sometimes fatal pseudomembranous colitis [4]. One of the main complications in treating CDI is the recurrence of the infection [5] defined as CDI which re-occurs within 8 weeks after the onset of a previous episode, provided the symptoms from the previous episode resolved after completion of initial treatment [6]. Most patients with an initial CDI episode respond to antibiotic treatment; however, up to 25% of patients may experience CDI recurrence within 30 days following treatment. Patients who experienced a recurrent episode of CDI have a 45% probability of a second recurrence, and this risk increases with each subsequent recurrence [1, 7]. Several risk factors have been identified for the development of recurrent CDI; however, certain subgroups of patients are more susceptible to recurrence such as patients of an older age, immunocompromised individuals those with a history of CDI or patients with an initial clinically severe CDI episode [8,9,10,11,12,13,14,15,16].
The economic burden of CDI is a result of extended length of hospital stay (LoS), re-admission, laboratory tests and medication. In Spain, the cost for treating C. difficile-associated diarrhoea (CDAD) was estimated at €32.2 million to the National Health System (NHS) in 2012 [17]. On average, the cost per episode was €3901, €4875 and €5916, for initial infection, first and second recurrence, respectively. The main extra CDI-associated costs were due to the extended LoS which was 7.4, 9.1 and 10.8 days for initial infection, first and second recurrence, respectively [17].
Bezlotoxumab is a new antitoxin agent (a human monoclonal antibody against C. difficile toxin B) which, administered during the course of antibiotic therapy for CDI, is indicated for the prevention of CDI recurrence in adults at high risk of CDI recurrence [18].
The phase III MODIFY I and MODIFY II trials [19] evaluated the efficacy and safety of a single intravenous infusion of bezlotoxumab compared to placebo in patients receiving standard of care (SoC) oral antibiotic therapy for CDI (metronidazole, vancomycin or fidaxomicin). Results from pooled data analysis showed a significantly lower rate of recurrent CDI with bezlotoxumab than placebo in all participants [16.5% (129 of 781) vs. 26.6% (206 of 773); adjusted difference, − 10.0 percentage points; 95% confidence interval (CI), − 14.0 to − 6.0; p value < 0.0001]. Moreover, the absolute difference in CDI recurrence rate between bezlotoxumab and placebo was greater in subpopulations at high risk of CDI recurrence than in the overall population.
Bezlotoxumab has been recently obtained the marketing authorization in Spain [20]. Although many studies have assessed the cost-effectiveness of antibiotic therapy for CDI [21], only one published cost-effectiveness analysis evaluating bezlotoxumab from a US payer perspective has been performed [22]. Due to the increasing burden of CDI and the efficacy of bezlotoxumab in reducing CDI recurrence, our aim was to assess the cost-effectiveness of bezlotoxumab in addition to SoC antibiotic therapy as compared to SoC alone for the prevention of recurrence in five subgroups of patients at high risk of CDI recurrence within the context of the Spanish NHS.
Methods
Model Overview
A computer-based Markov health state transition model was developed in Microsoft Excel® based on MODIFY I/II trials [19]. The model was previously adapted to the US setting [22] and for the present study the model was adapted to the Spanish context using local data.
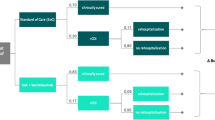
The model evaluated the cost-effectiveness of a single dose of bezlotoxumab 10 mg/kg administered as an intravenous infusion compared to placebo for the prevention of recurrence in five patient populations with CDI at increased recurrence risk according to MODIFY I/II trials [19]: (1) aged 65 years or older; (2) clinically severe CDI (defined as a Zar score ≥ 2; scores range from 1 to 8, with higher scores indicating more severe infection); (3) compromised immunity (determination made on the basis of medical history or use of immunosuppressive therapy for hematological malignancy—including leukemia, lymphoma, multiple myeloma—, an active malignancy requiring recent cytotoxic chemotherapy, receipt of a prior hematopoietic stem cell transplant, receipt of a prior solid organ transplant, asplenia, or neutropenia/pancytopenia due to other conditions); (4) a history of CDI (≥ 1 CDI episode in the previous 6 months); (5) aged 65 years or older and a history of CDI (≥ 1 CDI episode in the previous 6 months). The model structure is shown in Fig. 1. A cohort of patients entered the model with mild/moderate or a severe CDI episode. Patients with mild/moderate CDI could experience a clinical cure or a clinical failure. In addition, subjects with severe CDI could undergo colectomy surgery. Clinical cure and clinical failure were defined as per the MODIFY I/II trials [19]: (1) clinical cure: subject received ≤ 14-day regimen of SoC therapy and had no diarrhoea (≤ 2 loose stools per 24 h) for 2 consecutive days following completion of SoC therapy for the baseline CDI episode; and (2) clinical failure: subject required > 14-day regimen of SoC therapy for the baseline CDI episode.
Patients experiencing relief from CDI symptoms (clinical cure health state) can experience a mild/moderate or severe recurrence of CDI. Sustained response is achieved by those who remain in the clinical cure health state permanently without experiencing subsequent recurrence or death.
A patient experiencing clinical failure is treated further and eventually cured (post-clinical failure health state). In the current analysis, it was assumed that patients in post-clinical failure health state would not experience any recurrence of CDI since these patients were not followed in MODIFY I/II trials, and the efficacy of bezlotoxumab in addition to SoC was only assessed in those who had a clinical cure. Therefore, it was assumed that the consequences of these patients would not differ between treatment arms.
Patients who have experienced a colectomy move to a post-colectomy health state and are unable to experience a recurrence since CDI symptoms are characterized by colitis.
Death may occur in any health state.
The natural history of CDI is repeated for each recurrence, with a maximum of three possible recurrences.
Cycle Length, Time Horizon and Discount Rates
Given the acute nature of CDI illnesses and that the majority of recurrences occur within 30 days of initial infection, most of the costs are incurred within the first 6 months (180 days) following infection. Therefore, the cycle length was 15 days for the first 180 days, and annual thereafter.
The model assumed a lifetime horizon in order to ascertain the costs and benefits over the patient’s lifetime.
A 3% annual discount rate was adopted for both costs and benefits [23].
Model Inputs
Population characteristics (age at model entry, proportion of females, proportion of patients entering the model with severe CDI), first recurrence rate, severity of recurrence and efficacy of SoC were obtained from MODIFY I/II trials [24] for each subgroup (Table 1). Second and third recurrence rates, colectomy rates, mortality, costs and utilities were obtained from the literature (Table 2). Adverse events were not considered as the overall rates showed in MODIFY I/II trials were similar with bezlotoxumab and placebo [24].
Conservative assumptions were made if no data were available.
Recurrence
The rates of first recurrence for bezlotoxumab + SoC and SoC alone were taken from MODIFY I/II trials for each subgroup (Table 1). The duration of bezlotoxumab efficacy was assumed to be 12 weeks, corresponding to the follow-up period in the MODIFY I/II trials. Subsequent recurrence rates were based on a review of the literature on patients at high risk of recurrent CDI [25] and expert opinion (Table 2).
Efficacy of SoC
The clinical cure rates from the MODIFY I/II trials were used to estimate the efficacy of SoC for each subgroup (Table 1). The following assumptions were made: SoC efficacy at managing the index case was based on the data from subjects without a previous episode; SoC efficacy at managing the first recurrence was based on the data from subjects with one previous episode; and SoC efficacy at managing the subsequent recurrence was based on the data from subjects with two or more episodes.
Colectomy
The risk of colectomy and the mortality risk in patients requiring colectomy were based on a prospective cohort Spanish study [26], whose aim was to evaluate the frequency, associated risk factors and prognosis of first CDAD recurrences, and a literature review [27].
Mortality
The model assumes that the CDI-associated mortality risk is higher during the first 180 days after infection for patients who suffer a recurrence (36.3%) compared to those experiencing a sustained response (25.7%) based on Olsen et al. [28], who estimated the 6-month mortality in patients with recurrent CDI compared with patients with CDI who did not develop a recurrence in a retrospective cohort of hospitalized patients. Given the lack of evidence, it was assumed, based on Olsen et al. [28], that there is no CDI-associated mortality after 180 days; therefore, the mortality rate returns to that specified by the Spanish life tables [29] after that time.
Utility Values
Utility values for the different health states were derived from the published literature (Table 2). Wilcox et al. [30] conducted a retrospective study on resource use and health-related quality of life (HRQoL) associated with recurrent CDI hospitalizations in the UK. For the purpose of the model, the same utility value was assumed for health states “CDI mild/moderate”, “CDI severe”, “colectomy” and “clinical failure” based on Wilcox et al. [30]. The utility value for “post-colectomy” was obtained from a publication by Brown et al. [31], who investigated long-term HRQoL among patients who had undergone a colectomy within the previous 10 years. The baseline utility values from the general population were assumed to be applicable for “clinical cure” and “post-clinical failure” health states [32].
Costs
The cost inputs considered in the cost-effectiveness analysis were bezlotoxumab drug acquisition [20] and CDI episode (first, second and third recurrence) costs.
Consistent to MODIFY I/II trials [19], participants in both arms were receiving the same SoC (metronidazole, vancomycin and/or fidaxomicin) for 10–14 days; therefore the cost of SoC was not included as it would have no effect on the cost-effectiveness results.
The cost per episode of CDI was estimated based on Asensio et al. [17], who estimated the costs of CDI in Spain from the NHS perspective including antimicrobials (metronidazole, vancomycin), hospitalization, surgical procedures, measures to control the infection and recurrences of treated infections. It should be noted that the costs were estimated before the introduction of fidaxomicin, which is expensive compared to other SoC and often used for later recurrences. Therefore, the costs of managing recurrence may be underestimated leading to an underestimation of the cost savings associated with bezlotoxumab. This assumption was specifically addressed in a sensitivity analysis.
Costs were inflated to 2017 euros (€), where appropriate, using the Spanish consumer price index [33].
Analysis
Model Outputs
Model outcomes included the number of recurrences (first, second, third and total), the number needed to treat (NNT) to prevent one recurrence, 180-day mortality, life-years, quality-adjusted life years (QALYs) and costs for each treatment.
The cost-effectiveness analysis outcomes were expressed as the cost per recurrence avoided and cost per QALY gained. The incremental cost-effectiveness ratio (ICER) was estimated for bezlotoxumab added to SoC compared to SoC alone.
Sensitivity Analysis
Deterministic (DSA) and probabilistic sensitivity analysis (PSA) were performed in order to assess the impact of uncertainty on the ICER.
In DSA, the upper and lower bounds of the 95% confidence intervals were tested for each parameter (see Tables S1–S5 in the electronic supplementary material for details). When the confidence interval was not available or could not be estimated based on the literature, it was estimated assuming that the standard error was 5% of the base case value. Additionally, scenario analyses were performed in order to explore the variation of all utility’s values at the same time (extreme values) and the addition of the drug acquisition cost of fidaxomicin (Table 2) to CDI episode costs. Results of DSA were expressed as tornado charts.
PSA was performed with 1000 Monte Carlo simulations. The probabilities and utility values followed a beta distribution, costs followed a gamma distribution and for utility multipliers that have a base case value of 1, a triangular distribution with a mode of 1 was assumed (see Tables S6–S10 in the electronic supplementary material for details). Parameterization was based on the published literature where available. When no information on uncertainty could be sourced, the standard error was assumed to be 5% of the base case value. ICER scatter plots and cost-effectiveness acceptability curves were constructed for each subgroup.
The probability of bezlotoxumab being cost-effective at the €21, 000 per QALY gained willingness-to-pay (WTP) threshold recently established in Spain was estimated. Additionally, alternative thresholds at €30,000/QALY and €11,000/QALY were estimated as recommended specifically for sensitivity analysis [34].
Compliance with Ethics Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors.
Results
Base Case Scenario
Table 3 shows a summary of the results of the analysis. The addition of bezlotoxumab to SoC for the prevention of recurrence in patients at high risk of CDI recurrence compared to SoC alone was associated with a reduction in total recurrences and 180-day mortality and an increase in QALYs. The ICER was therefore €12,723.68, €17,494.70, €9544.72, €7386.38 and €4378.20 per QALY gained, for patients aged 65 years or older, clinically severe CDI, immunocompromised, with ≥ 1 CDI episode in the previous 6 months, and aged ≥ 65 years or older and ≥ 1 CDI episode in the pre vious 6 months, respectively.
Sensitivity Analysis
Deterministic Sensitivity Analysis
In the DSA, the model parameters that resulted in the highest degree of variability in the ICER were recurrence rate (first), all-cause mortality (180-days after CDI), and utility value for clinical cure health state (Fig. 2). In the subgroups of patients with ≥ 1 CDI episode in the previous 6 months and in patients aged ≥ 65 years and with ≥ 1 CDI episode in the previous 6 months, all analyses were below the €21,000 per QALY gained threshold.
Probabilistic Sensitivity Analysis
PSA results showed that bezlotoxumab was cost-effective at the €21,000 per QALY gained threshold for most of the simulations performed (Table 4). ICER scatter plots and cost-effectiveness acceptability curves for each subpopulation are presented in Figs. 3 and 4, respectively.
Discussion
Bezlotoxumab is the first and only drug indicated to prevent recurrences of CDI in high-risk patients. Therefore, its approach is different from antibiotics, which are licensed only to treat the infection. The model developed in the present study has shown that the addition of bezlotoxumab to SoC for the prevention of recurrence in five populations of patients at high risk of CDI recurrence was associated with an ICER below the €21,000 per QALY gained threshold [34] relative to SoC alone over a lifetime horizon from the perspective of the Spanish NHS. As expected, the lowest ICER (€4378.20 per QALY gained) was estimated for the subpopulation of patients aged 65 years or older with a history of CDI episodes, who achieved more health benefits, in terms of prevented recurrences and 180-day mortality, with a lower incremental cost of bezlotoxumab added to SoC compared to SoC alone. We also found that the probability of bezlotoxumab being cost-effective with a WTP of €21,000 per QALY gained [34] was high in all subgroups.
As mentioned before, a previous study evaluated the cost-effectiveness of bezlotoxumab added to SoC compared to SoC alone in six subgroups of patients at risk of CDI recurrence by using the same economic model [22]. Consistent with the US analysis, the present study showed similar ICER for the subgroups that were common between studies: ≥ 65 years of age ($15,298 vs. €12,723.68 per QALY gained), immunocompromised ($12,597 vs. €9544.72 per QALY gained), patients with a clinically severe CDI episode ($21,430 vs. €17,494.70 per QALY gained), and patients ≥ 65 years of age with ≥ 1 episodes of CDI within the previous 6 months ($3591 vs. €4378.20 per QALY gained). It should be noted that, according to DSA, the main parameters influencing cost-effectiveness results were recurrence rate (first) [19], 180-days mortality [28], and utility value for clinical cure health state [32], and our analysis used similar data to the US model for these parameters.
As with any model, there are some limitations in the present analysis, mainly related to input availability and model assumptions. First, in the absence of published studies measuring utility among patients with CDI, natural progression of disease or updated costs from the Spanish setting, some sources of the input parameters used were based on different populations and some assumptions reported in methods needed addressing. It should be noted that, although a higher recurrence rate after the second recurrence has been reported [25], the clinical experts assumed 45% should be applied to all subsequent recurrences as a conservative assumption. Further studies are required to obtain all these data. Second, regarding model structure, it was assumed that patients in post-clinical failure health state would not experience any recurrence of CDI since these patients were not tracked in MODIFY I/II trials. Although this might be seen as a conservative approach, as patients experiencing clinical failure would be treated and eventually cured, considering recurrences after clinical failure would lead to even higher benefits for those patients. Third, as most clinical input parameters of the model were based on MODIFY I/II trials, in which the evaluation of CDI severity was based on the Zar score, the proportion of patients with a severe CDI is probably underestimated, since more than 90% of participants were receiving SoC when scoring was performed [19]. Fourth, the cost of recurrence was based on a Spanish study which did not include fidaxomicin as it was conducted prior to its introduction. Fidaxomicin is expensive compared to vancomycin and metronidazole, and therefore the cost savings associated with bezlotoxumab may be underestimated. This point was addressed and confirmed in the DSA, which showed a lower cost associated with bezlotoxumab in the scenario analysis (addition of the drug acquisition cost of fidaxomicin to CDI episode costs) compared with the base case. ICER was 10.34, 6.17, 7.00, 10.49 and 29.63% lower for patients aged 65 years or older, clinically severe CDI, immunocompromised, with ≥ 1 CDI episode in the previous 6 months, and aged ≥ 65 years or older and ≥ 1 CDI episode in the previous 6 months, respectively, compared with the base case. On the other hand, it should also be noted that, in the MODIFY I/II trials, the population treated with fidaxomicin was small compared with that treated with vancomycin and metronidazole. Thus, if the population treated with fidaxomicin were larger, it could be expected that its greater efficacy (clinical cure) would have an effect on the results of cost-effectiveness. However, as stated in the model, the efficacy (clinical cure) of SoC is the same for both treatment and comparison arms and therefore an increase in the efficacy of SoC should not to have a significant effect on ICER. This fact has been equally addressed and confirmed by the DSA, showing minimal changes in ICER by varying the effectiveness of SoC in each subgroup. Fifth, the current analysis is unable to capture the transmission of the disease CDI. Patients who experience recurrent CDI have a high probability of repeating courses of antibiotic treatment, of adverse events as well as re-hospitalization, and acting as a reservoir of infection that can lead to secondary infection in other vulnerable patients [1]. Consequently, the ability of bezlotoxumab to prevent CDI recurrences should also have an impact on the overall incidence of CDI. Lastly, the cost-effectiveness analysis was carried out from the perspective of the Spanish NHS, which may underestimate the societal impact of the use of bezlotoxumab, such as time lost due to CDI or the cost of formal or informal caregivers.
Despite these limitations, a conservative approach has been adopted for this model, suggesting that the effectiveness of bezlotoxumab could be underestimated. Future investigations should address capturing the transmission of the disease, estimating the impact of the prevention of recurrence on the overall incidence of CDI and taking indirect costs and out-of-pocket costs incurred by the patient into account.
Although the findings reported in this study may be relevant to the Spanish healthcare system alone, approaches that can reduce CDI-associated resource use and costs should be of general interest [35], and the model can be adapted to other European countries.
Conclusions
In conclusion, the results suggest that bezlotoxumab added to SoC compared to SoC alone is a cost-effective treatment to prevent the recurrence of CDI in high-risk patients from the perspective of the Spanish NHS being the ICER below 21,000 per QALY gained in all subgroups. Deterministic sensitivity analysis showed results to be sensitive to variation in some parameters, specifically the recurrence rate (first) and all-causes mortality had a higher impact on the ICER in patients aged ≥ 65 years old, patients with severe CDI and patients with compromised immunity by establishing it above the threshold. Probabilistic sensitivity analysis determined that the probability that bezlotoxumab was cost-effective at a willingness-to-pay threshold of €21,000/QALY was 85.5, 54.1, 86.0, 94.5 and 99.6%, for patients aged 65 years or older, clinically severe CDI, immunocompromised, with ≥ 1 CDI episode in the previous 6 months, and aged ≥ 65 years or older and ≥ 1 CDI episode in the previous 6 months, respectively.
References
Bouza E. Consequences of Clostridium difficile infection: understanding the healthcare burden. Clin Microbiol Infect. 2012;18(6):5–12.
Davies KA, Longshaw CM, Davis GL, Bouza E, Barbut F, Barna Z, et al. Underdiagnosis of Clostridium difficile across Europe: the European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID). Lancet Infect Dis. 2014;14(12):1208–19.
Alcalá L, Reigadas E, Marín M, Martín A, Catalán P, Bouza E. Impact of clinical awareness and diagnostic tests on the underdiagnosis of Clostridium difficile infection. Eur J Clin Microbiol Infect Dis. 2015;34(8):1515–25.
Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431–55.
Bauer MP, Kuijper EJ, van Dissel JT. European Society of Clinical Microbiology and Infectious Diseases (ESCMID): treatment guidance document for Clostridium difficile infection (CDI). Clin Microbiol Infect. 2009;15(12):1067–79.
Debast SB, Bauer MP, Kuijper EJ, Allerberger F, Bouza E, Coia JE, et al. European society of clinical microbiology and infectious diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(S2):1–26.
Nagy E. What do we know about the diagnostics, treatment and epidemiology of Clostridioides (Clostridium) difficile infection in Europe? J Infect Chemother. 2018;24(3):164–70.
Garey KW, Sethi S, Yadav Y, DuPont HL. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect. 2008;70(4):298–304.
Abou Chakra CN, Pepin J, Sirard S, Valiquette L. Risk factors for recurrence, complications and mortality in Clostridium difficile infection: a systematic review. PLoS ONE. 2014;9(6):e98400.
Deshpande A, Pasupuleti V, Thota P, Pant C, Rolston DDK, Hernandez AV, et al. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36(04):452–60.
Mizusawa M, Doron S, Gorbach S. Clostridium difficile diarrhea in the elderly: current issues and management options. Drugs Aging. 2015;32(8):639–47.
Mittal C, Hassan S, Arshad S, Jeepalyam S, Bruni S, Miceli M, et al. Clostridium difficile infection in liver transplant recipients: a retrospective study of rates, risk factors and outcomes. Am J Transplant. 2014;14(8):1901–7.
Leav BA, Blair B, Leney M, Knauber M, Reilly C, Lowy I, et al. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI). Vaccine. 2010;28(4):965–9.
Bauer MP, Nibbering PH, Poxton IR, Kuijper EJ, van Dissel JT. Humoral immune response as predictor of recurrence in Clostridium difficile infection. Clin Microbiol Infect. 2014;20(12):1323–8.
Gupta SB, Mehta V, Dubberke ER, Zhao X, Dorr MB, Guris D, et al. Antibodies to toxin B are protective against Clostridium difficile infection recurrence. Clin Infect Dis. 2016;63(6):730–4.
Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. The role of toxin A and toxin B in Clostridium difficile infection. Nature. 2010;467(7316):711–3.
Asensio Á, Bouza E, Grau S, Rubio-rodríguez D, Rubio- C, Rubio-terrés C. Cost of Clostridium difficile associated diarrhea in Spain. Rev Esp Salud Publ. 2013;1:25–33.
European Medicines Agency (EMA). Summary of product characteristics. Zinplava (bezlotoxumab). 2017.
Wilcox MH, Gerding DN, Poxton IR, Kelly C, Nathan R, Birch T, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376(4):305–17.
Consejo General de Colegios Oficiales de Farmacéuticos. Bot Plus Web [Internet]. https://botplusweb.portalfarma.com/. Accessed 6 Oct 2017.
Burton HE, Mitchell SA, Watt M. A systematic literature review of economic evaluations of antibiotic treatments for Clostridium difficile infection. Pharmacoeconomics. 2017;35(11):1123–40.
Prabhu VS, Dubberke ER, Dorr MB, Elbasha E, Cossrow N, Jiang Y, et al. Cost-effectiveness of bezlotoxumab compared with placebo for the prevention of recurrent Clostridium difficile infection. Clin Infect Dis. 2018;66(3):355–62.
López-Bastida J, Oliva J, Antoñanzas F, García-Altés A, Gisbert R, Mar J, et al. Spanish recommendations on economic evaluation of health technologies. Eur J Heal Econ. 2010;11(5):513–20.
Clinical study reports of MODIFY I and II trials. Data on file. MSD.
Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18:21–7.
Larrainzar-Coghen T, Rodriguez-Pardo D, Puig-Asensio M, Rodríguez V, Ferrer C, Bartolomé R, et al. First recurrence of Clostridium difficile infection: clinical relevance, risk factors, and prognosis. Eur J Clin Microbiol Infect Dis. 2016;35(3):371–8.
Rodríguez-Pardo D, Mirelis B, Navarro F. Infecciones producidas por Clostridium difficile. Enferm Infecc Microbiol Clin. 2013;31(4):254–63.
Olsen MA, Yan Y, Reske KA, Zilberberg MD, Dubberke ER. Recurrent Clostridium difficile infection is associated with increased mortality. Clin Microbiol Infect. 2015;21(2):164–70.
Spanish National Statistics Institute. Mortality tables for the population of Spain 1991–2013 [Internet]. http://www.ine.es/jaxi/Tabla.htm?path=/t20/p319a/serie/p01/l0/&file=01001.px&L=0. Accessed 16 Oct 2017.
Wilcox MH, Ahir H, Coia JE, Dodgson A, Hopkins S, Llewelyn MJ, et al. Impact of recurrent Clostridium difficile infection: hospitalization and patient quality of life. J Antimicrob Chemother. 2017;72:2647–56.
Brown C, Gibson PR, Hart A, Kaplan GG, Kachroo S, Ding Q, et al. Long-term outcomes of colectomy surgery among patients with ulcerative colitis. Springerplus. 2015;4(1):573.
Szende A, Janssen B. Self-reported population health: an international perspective based on EQ-5D. Dordrecht: Springer; 2014.
Spanish National Statistics Institute. Consumer price index [Internet]. http://www.ine.es/calcula/calcula.do. Accessed 6 Oct 2017.
Ortega Eslava A, Marín Gil A, Fraga Fuentes M, López-Briz E, Puigventós Latorre F. Guía de evaluación económica e impacto presupuestario en los informes de evaluación de medicamentos. Guía práctica asociada al programa MADRE v 4.0. SEFH. 2016.
Reigadas Ramírez E, Bouza ES. Economic burden of Clostridium difficile infection in European Countries. In: Updates on Clostridium difficile in Europe. 2018. pp. 1–12.
Rodríguez-Pardo D, Almirante B, Bartolomé RM, Pomar V, Mirelis B, Navarro F, et al. Epidemiology of clostridium difficile infection and risk factors for unfavorable clinical outcomes: results of a hospital-based study in Barcelona, Spain. J Clin Microbiol. 2013;51(5):1465–73.
Acknowledgments
Funding
The study, article processing charges and open access fee were funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and/or Editorial Assistance
Medical writing assistance was provided by Susana Aceituno (Outcomes’10), which was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Miguel Salavert, Javier Cobo, Álvaro Pascual and Santiago Grau contributed to the validation of the model inputs and assumptions, analysis and interpretation of the results, and writing the manuscript. Belén Aragón, Stefano Maratia and Yiling Jiang contributed to the concept and design of the study. Susana Aceituno contributed to the coordination of the study, analysis and interpretation of the data, and writing the manuscript. All authors read and approved the final manuscript.
Prior Presentation
One abstract of this paper was presented at the 28th European Congress of Clinical Microbiology and Infectious Diseases (2018) as a poster presentation (E0164): cost-effectiveness of bezlotoxumab for the Prevention of recurrence of Clostridium difficile Infection in high risk patients in Spain.
Disclosures
Miguel Salavert has received payment for consultancy services for the submitted work from MSD Spain. Miguel Salavert has served as speaker for Janssen; and has received payment for lectures from MSD Spain and Pfizer. Javier Cobo has received payment for consultancy services for the submitted work from MSD Spain. Javier Cobo has received payment for lectures and has participate in advisory boards for Astellas Pharma and MSD Spain. Javier Cobo has received payment for consultancy services for the submitted work from MSD Spain. Álvaro Pascual has received payment for consultancy services for the submitted work from MSD Spain. Álvaro Pascual has participate in advisory boards for MSD Spain. Santiago Grau has received payment for consultancy services for the submitted work from MSD Spain. Santiago Grau has served as a consultant for Angelini Farmacéutica, Astellas Pharma and MSD Spain. Belén Aragón is an employee of MSD Spain and may own stock or stock options in the Company. Yiling Jiang is an employee of Merck Sharp & Dohme Ltd. UK and may own stock or stock options in the Company. Stefano Maratia is an employee of Chiltern International Spain, a contract research organization providing support to MSD Spain. Susana Aceituno works for an independent research institution which has received fees for its contribution to the project coordination as well as to the drafting of this manuscript. The sponsor had no influence on the evaluation of data, the preparation of the manuscript and the decision to submit it for publication.
Compliance with Ethics Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7165336.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Salavert, M., Cobo, J., Pascual, Á. et al. Cost-Effectiveness Analysis of Bezlotoxumab Added to Standard of Care Versus Standard of Care Alone for the Prevention of Recurrent Clostridium difficile Infection in High-Risk Patients in Spain. Adv Ther 35, 1920–1934 (2018). https://doi.org/10.1007/s12325-018-0813-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-018-0813-y