Abstract
Introduction
Complex mechanisms underlie dry eye (DE) symptom provocation. In particular, corneal hypersensitivity may provoke symptoms in short tear break-up time (BUT) DE characterized by tear film instability. We hypothesized that improved tear film stability may alleviate corneal sensitivity in patients with short tear BUT DE. Therefore, we investigated the effect of topical diquafosol tetrasodium (DQS) on corneal sensitivity in unstable tear film DE.
Methods
This prospective, randomized study included 27 subjects (age: 39.1 ± 8.4 years; range: 25–59 years) with short tear BUT DE, defined based on the presence of DE symptoms and tear film instability. Subjects were randomly divided into DQS (3% DQS, 12 subjects) and artificial tear (AT; preservative-free AT, 15 subjects) groups. Subjects applied the medication 6 times a day for 5 weeks. The perception of touch (S-touch) and pain (S-pain) sensitivity was measured using a Cochet—Bonnet esthesiometer. Tear evaluation, corneal sensitivity, and DE symptoms were compared before and after DQS or AT administration. The correlation between the improvement degrees of corneal sensitivity and DE symptoms following medication was analyzed.
Results
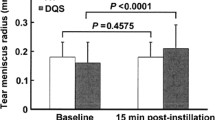
DQS significantly improved tear BUT and tear meniscus height (TMH) scores (p < 0.05), while AT significantly improved tear BUT (p < 0.05) but not TMH score. Mean S-pain and DE symptom scores were lower after medication use in the DQS (S-pain and DE symptoms: p < 0.05) and AT groups (S-pain: p = 0.05; DE symptoms: p < 0.05). However, S-touch did not change significantly in either group. A positive correlation was observed between the improvement degrees of S-pain and DE symptoms in the overall subjects studied.
Conclusion
Both DQS and AT alleviate corneal hypersensitivity and DE symptoms in eyes with short tear BUT DE. However, DQS seems to be more effective to adjust tear environment, leading to the normalization of corneal sensitivity and DE symptoms.
Trial Registration
UMIN Clinical Trials Registry Identifier, UMIN000014536



Similar content being viewed by others
References
The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international dry eye workshop (2007). Ocul Surf 2007;5:75–92.
Johnson ME. The association between symptoms of discomfort and signs in dry eye. Ocul Surf. 2009;7:199–211.
Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52:1938–78.
Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci. 2011;52:1994–2005.
Shimazaki-Den S, Dogru M, Higa K, Shimazaki J. Symptoms, visual function, and mucin expression of eyes with tear film instability. Cornea. 2013;32:1211–8.
Uchino Y, Uchino M, Yokoi N, et al. Alteration of tear mucin 5AC in office workers using visual display terminals: The Osaka Study. JAMA Ophthalmol. 2014;132:985–92.
Toda I, Fujishima H, Tsubota K. Ocular fatigue is the major symptom of dry eye. Acta Ophthalmol (Copenh). 1993;71:347–52.
Yokoi N, Uchino M, Uchino Y, et al. Importance of tear film instability in dry eye disease in office workers using visual display terminals: The Osaka Study. Am J Ophthalmol. 2015;159:748–54.
Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31:472–8.
Uchino M, Schaumberg DA, Dogru M, et al. Prevalence of dry eye disease among Japanese visual display terminal users. Ophthalmology. 2008;115:1982–8.
Uchino M, Yokoi N, Uchino Y, et al. Prevalence of dry eye disease and its risk factors in visual display terminal users: The Osaka Study. Am J Ophthalmol. 2013;156:759–66.
Kaido M, Kawashima M, Yokoi N, et al. Advanced dry eye screening for visual display terminal workers using functional visual acuity measurement: the Moriguchi study. Br J Ophthalmol. 2015;99:1488–92.
Rosenthal P, Borsook D. The corneal pain system. Part I: the missing piece of the dry eye puzzle. Ocul Surf. 2012;10:2–14.
Rosenthal P, Borsook D. Ocular neuropathic pain. Br J Ophthalmol. 2016;100:128–34.
Kaido M, Kawashima M, Ishida R, Tsubota K. Relationship of corneal pain sensitivity with dry eye symptoms in dry eye with short tear break-up time. Invest Ophthalmol Vis Sci. 2016;57:914–9.
Fujihara T, Murakami T, Fujita H, Nakamura M, Nakata K. Improvement of corneal barrier function by the P2Y(2) agonist INS365 in a rat dry eye model. Invest Ophthalmol Vis Sci. 2001;42:96–100.
Yokoi N, Kato H, Kinoshita S. Facilitation of tear fluid secretion by 3% diquafosol ophthalmic solution in normal human eyes. Am J Ophthalmol. 2014;157:85–92.
Jumblatt JE, Jumblatt MM. Regulation of ocular mucin secretion by P2Y2 nucleotide receptors in rabbit and human conjunctiva. Exp Eye Res. 1998;67:341–6.
Shigeyasu C, Yamada M, Akune Y, Tsubota K. Diquafosol sodium ophthalmic solution for the treatment of dry eye: clinical evaluation and biochemical analysis of tear composition. Jpn J Ophthalmol. 2015;59:415–20.
Kobashi H, Kamiya K, Igarashi A, Miyake T, Shimizu K. Intraocular scattering after instillation of diquafosol ophthalmic solution. Optom Vis Sci. 2015;92:e303–9.
Shimazaki-Den S, Iseda H, Dogru M, Shimazaki J. Effects of diquafosol sodium eye drops on tear film stability in short BUT type of dry eye. Cornea. 2013;32:1120–5.
Koh S, Ikeda C, Takai Y, Watanabe H, Maeda N, Nishida K. Long-term results of treatment with diquafosol ophthalmic solution for aqueous-deficient dry eye. Jpn J Ophthalmol. 2013;57:440–6.
Sakane Y, Yamaguchi M, Yokoi N, et al. Development and validation of the dry eye-related quality-of-life score questionnaire. JAMA Ophthalmol. 2013;131:1331–8.
van Bijsterveld OP. Diagnostic tests in the Sicca syndrome. Arch Ophthalmol. 1969;82:10–4.
Ibrahim OM, Dogru M, Ward SK, et al. The efficacy, sensitivity, and specificity of strip meniscometry in conjunction with tear function tests in the assessment of tear meniscus. Invest Ophthalmol Vis Sci. 2011;52:2194–8.
De Paiva CS, Pflugfelder SC. Corneal epitheliopathy of dry eye induces hyperesthesia to mechanical air jet stimulation. Am J Ophthalmol. 2004;137:109–15.
Bourcier T, Acosta MC, Borderie V, et al. Decreased corneal sensitivity in patients with dry eye. Invest Ophthalmol Vis Sci. 2005;46:2341–5.
Hoşal BM, Ornek N, Zilelioğlu G, Elhan AH. Morphology of corneal nerves and corneal sensation in dry eye: A Preliminary Study. Eye (Lond). 2005;19:1276–9.
Benítez del Castillo JM, Acosta MC, Wassfi MA, et al. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci. 2007;48:173–81.
Situ P, Simpson TL, Jones LW, Fonn D. Conjunctival and corneal hyperesthesia in subjects with dryness symptoms. Optom Vis Sci. 2008;85:867–72.
Spierer O, Felix ER, McClellan AL, et al. Corneal mechanical thresholds negatively associate with dry eye and ocular pain symptoms. Invest Ophthalmol Vis Sci. 2016;57:617–25.
Tsubota K, Yokoi N, Shimazaki J, et al. New perspectives on dry eye definition and diagnosis: a consensus report by the Asia Dry Eye Society. Ocul Surf. 2017;15:65–76.
Liu H, Begley C, Chen M, et al. A link between tear instability and hyperosmolarity in dry eye. Invest Ophthalmol Vis Sci. 2009;50:3671–9.
Belmonte C, Acosta MC, Schmelz M, Gallar J. Measurement of corneal sensitivity to mechanical and chemical stimulation with a CO2 esthesiometer. Invest Ophthalmol Vis Sci. 1999;40:513–9.
Acknowledgements
Funding
Facility provisions and equipment transport were funded by Santen Pharmaceutical Co., Ltd. The funding organization had no role in data analyses or in the conduct of this research. The article processing charges were funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Author Contribution statement
Conception and design of the study: Minako Kaido, Kazuo Tsubota. Analysis and interpretation of results: Minako Kaido. Manuscript preparation: Minako Kaido. Critical review of the article: Motoko Kawashima, Yoshiaki Yamada, Kazuo Tsubota. Data acquisition: Minako Kaido, Yuta Shigeno. Provision of materials, patients, or resources: Minako Kaido, Yoshiaki Yamada. Statistical expertise, literature searches: Minako Kaido. Administrative, technical, or logistic support: Minako Kaido, Kazuo Tsubota
Thanking Patient Participants
We thank all the study participants for their participation in the study.
Data Management
We wish to thank the Biostatistical Research Co. (Tokyo, Japan) for their assistance in data management.
Disclosures
Kazuo Tsubota is a consultant for Santen Pharmaceutical Co., Ltd. (Osaka, Japan). The Department of Ophthalmology, Keio University School of Medicine receives research funds from Santen Pharmaceutical Co., Ltd., and Kazuo Tsubota is a member of Santen’s Speaker’s Bureau. However, Santen Pharmaceutical Co., Ltd. was not involved with the scientific content of the research projects run by the department. Yoshiaki Yamada is an employee at Santen Pharmaceutical Co., Ltd. Minako Kaido, Motoko Kawashima and Yuta Shigeno have nothing to disclose.
Compliance with Ethics Guidelines
This study protocol was reviewed and approved by the Ethics Committee of the Institutional Review Board at the Shinanozaka Clinic. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study. This study was registered in the University hospital Medical Information Network (UMIN) (Registries No. UMIN000014536).
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to https://doi.org/10.6084/m9.figshare.5928670.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaido, M., Kawashima, M., Shigeno, Y. et al. Randomized Controlled Study to Investigate the Effect of Topical Diquafosol Tetrasodium on Corneal Sensitivity in Short Tear Break-Up Time Dry Eye. Adv Ther 35, 697–706 (2018). https://doi.org/10.1007/s12325-018-0685-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-018-0685-1