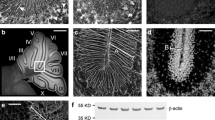
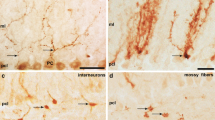
Abstract
Zebrin II/aldolase C expression in the normal cerebellum is restricted to a Purkinje cell subset and is the canonical marker for stripes and zones. This spatial restriction has been confirmed in over 30 species of mammals, birds, fish, etc. In a transgenic mouse model in which the Neurogenin 2 gene has been disrupted (Neurog2−/−), the cerebellum is smaller than normal and Purkinje cell dendrites are disordered, but the basic zone and stripe architecture is preserved. Here, we show that in the Neurog2−/− mouse, in addition to the normal Purkinje cell expression, zebrin II is also expressed in a population of cells with a morphology characteristic of microglia. This identity was confirmed by double immunohistochemistry for zebrin II and the microglial marker, Iba1. The expression of zebrin II in cerebellar microglia is not restricted by zone or stripe or lamina. A second zone and stripe marker, PLCβ4, does not show the same ectopic expression. When microglia are compared in control vs. Neurog2−/− mice, no difference is seen in apparent number or distribution, suggesting that the ectopic zebrin II immunoreactivity in Neurog2−/− cerebellum reflects an ectopic expression rather than the invasion of a new population of microglia from the periphery. This ectopic expression of zebrin II in microglia is unique as it is not seen in numerous other models of cerebellar disruption, such as in Acp2−/− mice and in human pontocerebellar hypoplasia. The upregulation of zebrin II in microglia is thus specific to the disruption of Neurog2 downstream pathways, rather than a generic response to a cerebellar disruption.






Similar content being viewed by others
References
Brochu G, Maler L, Zebrin HR II. A polypeptide antigen expressed selectively by Purkinje cells reveals compartments in rat and fish cerebellum. J Comp Neurol. 1990;291(4):538–52.
Eisenman LM, Hawkes R. Antigenic compartmentation in the mouse cerebellar cortex: zebrin and HNK-1 reveal a complex, overlapping molecular topography. J Comp Neurol. 1993;335(4):586–605.
Sillitoe RV, Marzban H, Larouche M, Zahedi S, Affanni J, Hawkes R. Conservation of the architecture of the anterior lobe vermis of the cerebellum across mammalian species. Prog Brain Res. 2005;148:283–97.
Pakan JM, Iwaniuk AN, Wylie DR, Hawkes R, Marzban H. Purkinje cell compartmentation as revealed by zebrin II expression in the cerebellar cortex of pigeons (Columba livia). J Comp Neurol. 2007;501(4):619–30.
Marzban H, Hawkes R. On the architecture of the posterior zone of the cerebellum. Cerebellum. 2011;10(3):422–34.
Marzban H, Zahedi S, Sanchez M, Hawkes R. Antigenic compartmentation of the cerebellar cortex in the Syrian hamster Mesocricetus auratus. Brain Res. 2003;974(1):176–83.
Kim JY, Marzban H, Chung SH, Watanabe M, Eisenman LM, Hawkes R. Purkinje cell compartmentation of the cerebellum of microchiropteran bats. J Comp Neurol. 2009;517(2):193–209.
Sillitoe RV, Malz CR, Rockland K, Hawkes R. Antigenic compartmentation of the primate and tree shrew cerebellum: a common topography of zebrin II in Macaca mulatta and Tupaia belangeri. J Anat. 2004;204(4):257–69.
Staugaitis SM, Zerlin M, Hawkes R, Levine JM, Goldman JE. Aldolase C/zebrin II expression in the neonatal rat forebrain reveals cellular heterogeneity within the subventricular zone and early astrocyte differentiation. J Neurosci Off J Soc Neurosci. 2001;21(16):6195–205.
Caffe AR, Von Schantz M, Szel A, Voogd J, Van Veen T. Distribution of Purkinje cell-specific Zebrin-II/aldolase C immunoreactivity in the mouse, rat, rabbit, and human retina. J Comp Neurol. 1994;348(2):291–7.
Popovici T, Berwald-Netter Y, Vibert M, Kahn A, Skala H. Localization of aldolase C mRNA in brain cells. FEBS Lett. 1990;268(1):189–93.
Fode C, Ma Q, Casarosa S, Ang SL, Anderson DJ, Guillemot F. A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes Dev. 2000;14(1):67–80.
Parras CM, Schuurmans C, Scardigli R, Kim J, Anderson DJ, Guillemot F. Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity. Genes Dev. 2002;16(3):324–38.
Schuurmans C, Armant O, Nieto M, Stenman JM, Britz O, Klenin N, et al. Sequential phases of cortical specification involve Neurogenin-dependent and -independent pathways. EMBO J. 2004;23(14):2892–902.
Salsano E, Croci L, Maderna E, Lupo L, Pollo B, Giordana MT, et al. Expression of the neurogenic basic helix-loop-helix transcription factor NEUROG1 identifies a subgroup of medulloblastomas not expressing ATOH1. Neuro-oncology. 2007;9(3):298–307.
Zordan P, Croci L, Hawkes R, Consalez GG. Comparative analysis of proneural gene expression in the embryonic cerebellum. Dev Dyn Off Publ Am Assoc Anatomists. 2008;237(6):1726–35.
Henke RM, Savage TK, Meredith DM, Glasgow SM, Hori K, Dumas J, et al. Neurog2 is a direct downstream target of the Ptf1a-Rbpj transcription complex in dorsal spinal cord. Development (Cambridge, England). 2009;136(17):2945–54.
Florio M, Leto K, Muzio L, Tinterri A, Badaloni A, Croci L, et al. Neurogenin 2 regulates progenitor cell-cycle progression and Purkinje cell dendritogenesis in cerebellar development. Development (Cambridge, England). 2012;139(13):2308–20.
Figueiredo C, Pais TF, Gomes JR, Chatterjee S. Neuron-microglia crosstalk up-regulates neuronal FGF-2 expression which mediates neuroprotection against excitotoxicity via JNK1/2. J Neurochem. 2008;107(1):73–85.
Parkhurst CN, Gan WB. Microglia dynamics and function in the CNS. Curr Opin Neurobiol. 2010;20(5):595–600.
Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation. Glia. 2013;61(1):71–90.
Mrak RE, Griffin WS. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging. 2005;26(3):349–54.
Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, et al. Role of microglia in central nervous system infections. Clin Microbiol Rev. 2004;17(4):942–64. table of contents
Wong EL, Stowell RD, Majewska AK. What the Spectrum of microglial functions can teach us about fetal alcohol Spectrum disorder. Front Synaptic Neurosci. 2017;9:11.
Marin-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41(4):535–47.
Polazzi E, Gianni T, Contestabile A. Microglial cells protect cerebellar granule neurons from apoptosis: evidence for reciprocal signaling. Glia. 2001;36(3):271–80.
Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1(2):129–43.
Ma Q, Fode C, Guillemot F, Anderson DJ. Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev. 1999;13(13):1717–28.
Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20(3):469–82.
Mattar P, Britz O, Johannes C, Nieto M, Ma L, Rebeyka A, et al. A screen for downstream effectors of Neurogenin2 in the embryonic neocortex. Dev Biol. 2004;273(2):373–89.
Bailey K, Balaei MR, Mannan A, Del Bigio MR, Marzban H. Purkinje cell compartmentation in the cerebellum of the lysosomal acid phosphatase 2 mutant mouse (nax-naked-ataxia mutant mouse). PLoS One. 2014;9(4):e94327.
Bailey K, Balaei MR, Mehdizadeh M, Marzban H. Spatial and temporal expression of lysosomal acid phosphatase 2 (ACP2) reveals dynamic patterning of the mouse cerebellar cortex. Cerebellum. 2013;12(6):870–81.
Chung SH, Marzban H, Aldinger K, Dixit R, Millen K, Schuurmans C, et al. Zac1 plays a key role in the development of specific neuronal subsets in the mouse cerebellum. Neural Dev. 2011;6:25.
Hazama GI, Yasuhara O, Morita H, Aimi Y, Tooyama I, Kimura H. Mouse brain IgG-like immunoreactivity: strain-specific occurrence in microglia and biochemical identification of IgG. J Comp Neurol. 2005;492(2):234–49.
Sillitoe RV, Hawkes R. Whole-mount immunohistochemistry: a high-throughput screen for patterning defects in the mouse cerebellum. J Histochem Cytochem Off J Histochem Soc. 2002;50(2):235–44.
Wilkinson G, Dennis D, Schuurmans C. Proneural genes in neocortical development. Neuroscience. 2013;253:256–73.
Sarna JR, Marzban H, Watanabe M, Hawkes R. Complementary stripes of phospholipase Cbeta3 and Cbeta4 expression by Purkinje cell subsets in the mouse cerebellum. J Comp Neurol. 2006;496(3):303–13.
Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Mol Brain Res. 1998;57(1):1–9.
Vedeler C, Ulvestad E, Grundt I, Conti G, Nyland H, Matre R, et al. Fc receptor for IgG (FcR) on rat microglia. J Neuroimmunol. 1994;49(1):19–24.
Saftig P, Hartmann D, Lullmann-Rauch R, Wolff J, Evers M, Koster A, et al. Mice deficient in lysosomal acid phosphatase develop lysosomal storage in the kidney and central nervous system. J Biol Chem. 1997;272(30):18628–35.
Rahimi Balaei M, Jiao X, Ashtari N, Afsharinezhad P, Ghavami S, Marzban H. Cerebellar expression of the Neurotrophin receptor p75 in naked-ataxia mutant mouse. Int J Mol Sci. 2016;17(1).
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–5.
Eisenman LM, Gallagher E, Hawkes R. Regionalization defects in the weaver mouse cerebellum. J Comp Neurol. 1998;394(4):431–44.
Tano D, Napieralski JA, Eisenman LM, Messer A, Plummer J, Hawkes R. Novel developmental boundary in the cerebellum revealed by zebrin expression in the lurcher (Lc/+) mutant mouse. J Comp Neurol. 1992;323(1):128–36.
Hawkes R, Herrup K, Aldolase C. Zebrin II and the regionalization of the cerebellum. J Mol Neurosci. 1995;6(3):147–58.
Howell BW, Hawkes R, Soriano P, Cooper JA. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature. 1997;389(6652):733–7.
Gallagher E, Howell BW, Soriano P, Cooper JA, Hawkes R. Cerebellar abnormalities in the disabled (mdab1–1) mouse. J Comp Neurol. 1998;402(2):238–51.
Edwards MA, Leclerc N, Crandall JE, Yamamoto M. Purkinje cell compartments in the reeler mutant mouse as revealed by Zebrin II and 90-acetylated glycolipid antigen expression. Anat Embryol. 1994;190(5):417–28.
Goldowitz D, Cushing RC, Laywell E, D’Arcangelo G, Sheldon M, Sweet HO, et al. Cerebellar disorganization characteristic of reeler in scrambler mutant mice despite presence of reelin. J Neurosci. 1997;17(22):8767–77.
Sarna J, Miranda SR, Schuchman EH, Hawkes R. Patterned cerebellar Purkinje cell death in a transgenic mouse model of Niemann pick type a/B disease. Eur J Neurosci. 2001;13(10):1873–80.
Sarna JR, Larouche M, Marzban H, Sillitoe RV, Rancourt DE, Hawkes R. Patterned Purkinje cell degeneration in mouse models of Niemann-pick type C disease. J Comp Neurol. 2003;456(3):279–91.
Ji Z, Hawkes R. Partial ablation of the neonatal external granular layer disrupts mossy fiber topography in the adult rat cerebellum. J Comp Neurol. 1996;371(4):578–88.
McCORMICK DA, Steinmetz JE, Thompson RF. Lesions of the inferior olivary complex cause extinction of the classically conditioned eyeblink response. Brain Res. 1985;359(1–2):120–30.
Ashwell K. Microglia and cell death in the developing mouse cerebellum. Dev Brain Res. 1990;55(2):219–30.
Kovach C, Dixit R, Li S, Mattar P, Wilkinson G, Elsen GE, et al. Neurog2 simultaneously activates and represses alternative gene expression programs in the developing neocortex. Cereb Cortex. 2013;23(8):1884–900.
Sun Y, Nadal-Vicens M, Misono S, Lin MZ, Zubiaga A, Hua X, et al. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell. 2001;104(3):365–76.
Fletcher CF, Lutz CM, O'Sullivan TN, Shaughnessy JD, Hawkes R, Frankel WN, et al. Absence epilepsy in tottering mutant mice is associated with calcium channel defects. Cell. 1996;87(4):607–17.
Leclerc N, Schwarting GA, Herrup K, Hawkes R, Yamamoto M. Compartmentation in mammalian cerebellum: Zebrin II and P-path antibodies define three classes of sagittally organized bands of Purkinje cells. Proc Natl Acad Sci. 1992;89(11):5006–10.
Sawada K, Fukui Y. Expression of tyrosine hydroxylase in cerebellar Purkinje cells of ataxic mutant mice: its relation to the onset and/or development of ataxia. J Med Invest. 2001;48(1/2):5–10.
Acknowledgments
These studies were supported by grants from the Children Hospital Research Institute of Manitoba (HM). We are grateful to Carol Schuurmans for providing ngn2 knockout mice, and Marc Del Bigio for providing slides of pontocerebellar hypoplasia and normal control section samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Electronic Supplementary Material
Fig. Suppl. 1
. A) Acp2−/− and wt sibling cerebellum at P5 and P7 (P5; n = 1 and P7; n = 2) were isolated and extracted total RNA was shipped to the McGill University and Genome Quebec Innovation Centre (MUGQIC). The raw RNA-sequencing data were trimmed and clipped with STAR (Spliced Transcripts Alignment to a Reference) program. RNA-sequencing data analysis indicates that the Neurog2 expression upregulated in Acp2−/− cerebella. B) In order to quantify microglia in cerebellum of the Neurog2−/− and wt, we counted microglia immunostained with Iba1 in six sections through the vermis of P15 animals, capturing 5 areas in each zone (each area = 0.6 mm*0.64 mm). We calculated microglia number to be: 59.69 ± 1.36 per 1mm2 in wild-type cerebella and 55.94 ± 1.28/1mm2 in Neurog2−/− cerebella (mean ± SE). There were no significant differences between wild-type and Neurog2−/− cerebella. (PNG 1.02 mb)
Rights and permissions
About this article
Cite this article
Rahimi-Balaei, M., Jiao, X., Shabanipour, S. et al. Zebrin II Is Ectopically Expressed in Microglia in the Cerebellum of Neurogenin 2 Null Mice. Cerebellum 18, 56–66 (2019). https://doi.org/10.1007/s12311-018-0944-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-018-0944-3