Abstract
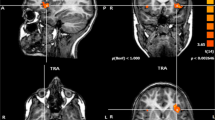
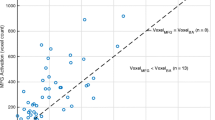
The cerebellum is known for its crossed activation pattern with the contralateral cerebral hemisphere during language functional magnetic resonance imaging (fMRI) tasks in healthy patients. Crossed cerebro-cerebellar activation has been previously shown to occur in patients with brain tumors not affecting the activation areas. However, the presence of a tumor in left Broca’s area in the inferior frontal gyrus is known to disrupt cerebral activation during language tasks. This study investigated if crossed cerebro-cerebellar activation patterns for language tasks would still occur in such patients. A total of 43 right-handed patients with a glioma affecting left Broca’s area were examined for their cerebral and cerebellar activation during an fMRI language task. Only 13 of the 43 patients exhibited crossed cerebro-cerebellar activation patterns. Statistically significant differences of atypical cerebro-cerebellar activation patterns were found between cerebral right-dominant (RD) and cerebral co-dominant (CD) (p < 0.001) as well as cerebral RD and cerebral left-dominant (LD) patients (p < 0.01), while no differences were found when patients were divided based on cerebellar dominance (p > 0.75) or tumor grade (p > 0.5). No relation was found between the cerebellar and cerebral laterality index (LI) values (ρ = − 0.20; p = 0.21). Atypical activation patterns are suspected to have been caused by the tumor, perhaps a result of contralateral reorganization in some cases and false negative activation in left Broca’s area from neurovascular uncoupling (NVU) in others. Cerebellar activation may also potentially indicate cerebral false negative behavior and future cerebral contralateral reorganization.


Similar content being viewed by others
Change history
03 October 2019
The original version of this article unfortunately contained mistake in Funding information section.
03 October 2019
The original version of this article unfortunately contained mistake in Funding information section.
References
Hubrich-Ungureanu P, Kaemmerer N, Henn FA, Braus DF. Lateralized organization of the cerebellum in a silent verbal fluency task: a functional magnetic resonance imaging study in healthy volunteers. Neurosci Lett. 2002;319(2):91–4. https://doi.org/10.1016/S0304-3940(01)02566-6.
Jansen A, Flöel A, Van Randenborgh J, Konrad C, Rotte M, Förster A-F, et al. Crossed cerebro–cerebellar language dominance. Hum Brain Mapp. 2005;24(3):165–72. https://doi.org/10.1002/hbm.20077.
Riecker A, Ackermann H, Wildgruber D, Dogil G, Grodd W. Opposite hemispheric lateralization effects during speaking and singing at motor cortex, insula and cerebellum. Neuroreport. 2000;11(9):1997–2000. https://doi.org/10.1097/00001756-200006260-00038.
Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage. 2011;59(2):1560–70. https://doi.org/10.1016/j.neuroimage.2011.08.065.
Lux S, Keller S, Mackay C, Ebers G, Marshall JC, Cherkas L, et al. Crossed cerebral lateralization for verbal and visuo-spatial function in a pair of handedness discordant monozygotic twins: MRI and fMRI brain imaging. J Anat. 2008;212(3):235–48. https://doi.org/10.1111/j.1469-7580.2008.00855.x.
Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997;17(1):353–62.
Isaacs KL, Barr WB, Nelson PK, Devinsky O. Degree of handedness and cerebral dominance. Neurology. 2006;66(12):1855–8. https://doi.org/10.1212/01.wnl.0000219623.28769.74.
Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, et al. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123(12):2512–8. https://doi.org/10.1093/brain/123.12.2512.
Gelinas JN, Fitzpatrick KP, Kim HC, Bjornson BH. Cerebellar language mapping and cerebral language dominance in pediatric epilepsy surgery patients. NeuroImage: Clinical. 2014;6:296–306. https://doi.org/10.1016/j.nicl.2014.06.016.
Connor LT, DeShazo Braby T, Snyder AZ, Lewis C, Blasi V, Corbetta M. Cerebellar activity switches hemispheres with cerebral recovery in aphasia. Neuropsychologia. 2006;44(2):171–7. https://doi.org/10.1016/j.neuropsychologia.2005.05.019.
Lidzba K, Wilke M, Staudt M, Krageloh-Mann I, Grodd W. Reorganization of the cerebro-cerebellar network of language production in patients with congenital left-hemispheric brain lesions. Brain Lang. 2008;106(3):204–10. https://doi.org/10.1016/j.bandl.2007.11.003.
Mendez Orellana C, Visch-Brink E, Vernooij M, Kalloe S, Satoer D, Vincent A, et al. Crossed cerebrocerebellar language lateralization: an additional diagnostic feature for assessing atypical language representation in presurgical functional MR imaging. Am J Neuroradiol. 2015;36(3):518–24. https://doi.org/10.3174/ajnr.A4147.
Duffau H. Brain mapping: from neural basis of cognition to surgical applications. Springer. 2011, https://doi.org/10.1007/978-3-7091-0723-2.
Partovi S, Jacobi B, Rapps N, Zipp L, Karimi S, Rengier F, et al. Clinical standardized fMRI reveals altered language lateralization in patients with brain tumor. Am J Neuroradiol. 2012;33(11):2151–7. https://doi.org/10.3174/ajnr.A3137.
Thiel A, Habedank B, Winhuisen L, Herholz K, Kessler J, Haupt WF, et al. Essential language function of the right hemisphere in brain tumor patients. Ann Neurol. 2005;57(1):128–31. https://doi.org/10.1002/ana.20342.
Tantillo G, Peck KK, Arevalo-Perez J, Lyo JK, Chou JF, Young RJ, et al. Corpus callosum diffusion and language lateralization in patients with brain tumors: a DTI and fMRI study. J Neuroimaging. 2015;26(2):224–31. https://doi.org/10.1111/jon.12275.
Kurabe S, Itoh K, Nakada T, Fujii Y. Evidence for cerebellar motor functional reorganization in brain tumor patients: an fMRI study. Neurosci Lett. 2016;622:45–8. https://doi.org/10.1016/j.neulet.2016.04.036.
Fraga de Abreu VH, Peck KK, Petrovich-Brennan NM, Woo KM, Holodny AI. Brain tumors: the influence of tumor type and routine MR imaging characteristics at BOLD functional MR imaging in the primary motor gyrus. Radiology. 2016.
Hou BL, Bradbury M, Peck KK, Petrovich NM, Gutin PH, Holodny AI. Effect of brain tumor neovasculature defined by rCBV on BOLD fMRI activation volume in the primary motor cortex. NeuroImage. 2006;32(2):489–97. https://doi.org/10.1016/j.neuroimage.2006.04.188.
Holodny AI, Schulder M, Liu WC, Wolko J, Maldjian JA, Kalnin AJ. The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: implications for image-guided neurosurgery. Am J Neuroradiol. 2000;21(8):1415–22.
Zaca D, Jovicich J, Nadar SR, Voyvodic JT, Pillai JJ. Cerebrovascular reactivity mapping in patients with low grade gliomas undergoing presurgical sensorimotor mapping with BOLD fMRI. J Magn Reson Imaging. 2014;40(2):383–90. https://doi.org/10.1002/jmri.24406.
Ulmer JL, Hacein-Bey L, Mathews VP, Mueller WM, DeYoe EA, Prost RW, et al. Lesion-induced pseudo-dominance at functional magnetic resonance imaging: implications for preoperative assessments. Neurosurgery. 2004;55(3):569–79. https://doi.org/10.1227/01.NEU.0000134384.94749.B2.
Dong JW, Brennan NMP, Izzo G, Peck KK, Holodny AI. fMRI activation in the middle frontal gyrus as an indicator of hemispheric dominance for language in brain tumor patients: a comparison with Broca’s area. Neuroradiology. 2016;58(5):513–20. https://doi.org/10.1007/s00234-016-1655-4.
Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. https://doi.org/10.1016/0028-3932(71)90067-4.
Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–73. https://doi.org/10.1006/cbmr.1996.0014.
Peck KK, Bradbury M, Petrovich NM, Hou BL, Ishill N, Brennan C, et al. Presurgical evaluation of language using functional magnetic resonance imaging in brain tumor patients with previous surgery. Neurosurgery. 2009;64(4):644–53. https://doi.org/10.1227/01.NEU.0000339122.01957.0A.
Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21(2):700–12.
Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19(10):2485–97. https://doi.org/10.1093/cercor/bhp135.
Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp. 1996;4(3):174–98. https://doi.org/10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0.
Ulmer JL, Krouwer HG, Mueller WM, Ugurel MS, Kocak M, Mark LP. Pseudo-reorganization of language cortical function at fMR imaging: a consequence of tumor-induced neurovascular uncoupling. Am J Neuroradiol. 2003;24(2):213–7.
Seghier ML. Laterality index in functional MRI: methodological issues. Magn Reson Imaging. 2008;26(5):594–601. https://doi.org/10.1016/j.mri.2007.10.010.
Acknowledgements
We would like to thank Joanne Chin for her editorial assistance.
Funding
This research was funded in part through the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
For this type of study, formal consent is not required. A waiver of informed consent was issued by our institutional review board. This article does not contain any studies with animals performed by any of the authors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cho, N.S., Peck, K.K., Zhang, Z. et al. Paradoxical Activation in the Cerebellum During Language fMRI in Patients with Brain Tumors: Possible Explanations Based on Neurovascular Uncoupling and Functional Reorganization. Cerebellum 17, 286–293 (2018). https://doi.org/10.1007/s12311-017-0902-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-017-0902-5