Abstract
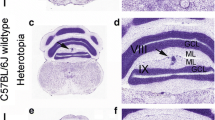
The cerebellar vermis is particularly vulnerable to neurodevelopmental malformations in humans and rodents. Sprague–Dawley, and Long–Evans rats exhibit spontaneous cerebellar malformations consisting of heterotopic neurons and glia in the molecular layer of the vermis. Malformations are almost exclusively found along the primary fissure and are indicative of deficits of neuronal migration during cerebellar development. In the present report, we test the prediction that genetically engineered rats on Sprague–Dawley or Long–Evans backgrounds will also exhibit the same cerebellar malformations. Consistent with our hypothesis, we found that three different transgenic lines on two different backgrounds had cerebellar malformations. Heterotopia in transgenic rats had identical cytoarchitecture as that observed in wild-type rats including altered morphology of Bergmann glia. In light of the possibility that heterotopia could affect results from behavioral studies, these data suggest that histological analyses be performed in studies of cerebellar function or development when using genetically engineered rats on these backgrounds in order to have more careful interpretation of experimental findings.


Similar content being viewed by others
References
Nilsen LH, Witter MP, Sonnewald U (2014) Neuronal and astrocytic metabolism in a transgenic rat model of Alzheimer’s disease. J Cereb Blood Flow Metab: 1–9. Available: http://www.ncbi.nlm.nih.gov/pubmed/24594625.
Iulita MF, Allard S, Richter L, Munter L-M, Ducatenzeiler A, et al. (2014) Intracellular Abeta pathology and early cognitive impairments in a transgenic rat model overexpressing human amyloid precursor protein: a multidimensional study. Acta Neuropathol Commun 2: 61. Available: http://www.ncbi.nlm.nih.gov/pubmed/24903713.
Lejavova K, Ondicova K, Horvathova L, Hegedusova N, Cubinkova V, et al. (2014) Stress-induced activation of the sympathoadrenal system is determined by genetic background in rat models of tauopathy. J Alzheimer’s Dis. Available: http://www.ncbi.nlm.nih.gov/pubmed/25147110. Accessed 3 October 2014
Hamilton SM, Green JR, Veeraragavan S, Yuva L, McCoy A, et al. Fmr1 and Nlgn3 knockout rats: novel tools for investigating autism spectrum disorders. Behav Neurosci. 2014;128:103–9. http://www.ncbi.nlm.nih.gov/pubmed/24773431.
Dave KD, De Silva S, Sheth NP, Ramboz S, Beck MJ, et al. Phenotypic characterization of recessive gene knockout rat models of Parkinson’s disease. Neurobiol Dis. 2014;70:190–203. doi:10.1016/j.nbd.2014.06.009.
Sun J, Kouranova E, Cui X, Mach RH, Xu J. Regulation of dopamine presynaptic markers and receptors in the striatum of DJ-1 and pink1 knockout rats. Neurosci Lett. 2013;557:123–8. doi:10.1016/j.neulet.2013.10.034.
Lee J-W, Tapias V, Di Maio R, Greenamyre JT, Cannon JR (2014) Behavioral, neurochemical, and pathologic alterations in bacterial artificial chromosome transgenic G2019S leucine-rich repeated kinase 2 rats. Neurobiol Aging. Available: http://www.ncbi.nlm.nih.gov/pubmed/25174649. Accessed 7 September 2014
Daher JPL, Volpicelli-Daley LA, Blackburn JP, Moehle MS, West AB. Abrogation of α-synuclein-mediated dopaminergic neurodegeneration in LRRK2-deficient rats. Proc Natl Acad Sci U S A. 2014;111:9289–94. http://www.ncbi.nlm.nih.gov/pubmed/24927544.
Rossignol J, Fink K, Davis K, Clerc S, Crane A, et al. Transplants of adult mesenchymal and neural stem cells provide neuroprotection and behavioral sparing in a transgenic rat model of Huntington’s disease. Stem Cells. 2014;32:500–9. doi:10.1002/stem.1508.
Urbach YK, Raber KA, Canneva F, Plank AC, Andreasson T, et al. Automated phenotyping and advanced data mining exemplified in rats transgenic for Huntington’s disease. J Neurosci Methods. 2014;234:38–53. doi:10.1016/j.jneumeth.2014.06.017.
Riva N, Chaabane L, Peviani M, Ungaro D, Domi T, et al. Defining peripheral nervous system dysfunction in the SOD-1G93A transgenic rat model of amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2014;73:658–70. http://www.ncbi.nlm.nih.gov/pubmed/24918640.
Halon M, Kaczor JJ, Ziolkowski W, Flis DJ, Borkowska A, et al. Changes in skeletal muscle iron metabolism outpace amyotrophic lateral sclerosis onset in transgenic rats bearing the G93A hmSOD1 gene mutation. Free Radic Res. 2014;48:1363–70. http://www.ncbi.nlm.nih.gov/pubmed/25175826. Accessed 3 October 2014.
Kelp A, Koeppen AH, Petrasch-parwez E, Calaminus C, Bauer C, et al. A novel transgenic rat model for spinocerebellar ataxia type 17 recapitulates neuropathological changes and supplies in vivo imaging biomarkers. J Neurosci. 2013;33:9068–81. http://www.ncbi.nlm.nih.gov/pubmed/23699518.
Brown AJ, Fisher DA, Kouranova E, McCoy A, Forbes K, et al. Whole-rat conditional gene knockout via genome editing. Nat Methods. 2013;10:638–40. http://www.ncbi.nlm.nih.gov/pubmed/23749298.
Pan Y. A new tool to generate transgenic rats using female germline stem cells from post-natal ovaries. Mol Hum Reprod. 2014;20:283–5. http://www.ncbi.nlm.nih.gov/pubmed/24608712. Accessed 3 Oct 2014.
Reichardt HM, Fischer HJ (2014) Generation of transgenic rats using lentiviral vectors. Methods Mol Biol. Available: http://www.ncbi.nlm.nih.gov/pubmed/25063498. Accessed 3 Oct 2014
Ma Y, Ma J, Zhang X, Chen W, Yu L, et al. Generation of eGFP and Cre knockin rats by CRISPR/Cas9. FEBS J. 2014. doi:10.1111/febs.12935.
Necchi D, Scherini E. The malformation of the cerebellar fissura prima: a tool for studying histogenetic processes. Cerebellum. 2002;1:137–42. doi:10.1080/147342202753671277.
Necchi D, Soldani C, Bernocchi G, Scherini E. Development of the anatomical alteration of the cerebellar fissura prima. Anat Rec. 2000;259:150–6. doi:10.1002/(SICI)1097-0185(20000601)259:2<150::AID-AR5>3.0.CO;2-A.
Griffin WST, Eriksson MAE, Del Cerro M, Woodward DJ, Stampfer N. Naturally occurring alterations of cortical layers surrounding the fissura prima of rat cerebellum. J Comp Neurol. 1980;192:109–18. doi:10.1002/cne.901920107.
Ezerman EB, Kromer LF. Outbred Sprague–Dawley rats from two breeders exhibit different incidences of neuroanatomical abnormalities affecting the primary cerebellar fissure. Exp Brain Res. 1985;59:625–8. doi:10.1007/BF00261354.
Cerri S, Piccolini VM, Bernocchi G. Postnatal development of the central nervous system: anomalies in the formation of cerebellum fissures. Anat Rec. 2010;293:492–501. doi:10.1002/ar.21082.
Van Dine SE, Salem E, George E, Siu NY, Dotzler T, Ramos RL. Cellular and axonal diversity in molecular layer heterotopia of the rat cerebellar vermis. Biomed Res Int. 2013;2013:805467.
Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–33. doi:10.1016/j.neuron.2011.10.028.
Schonig K, Weber T, Frommig A, Wendler L, Pesold B, et al. Conditional gene expression systems in the transgenic rat brain. BMC Biol. 2012;10:77. doi:10.1186/1741-7007-10-77.
Weber T, Schönig K, Tews B, Bartsch D (2011) Inducible gene manipulations in brain serotonergic neurons of transgenic rats. PLoS One 6. doi:10.1371/journal.pone.0028283
Ramos RL, Smith PT, DeCola C, Tam D, Corzo O, et al. Cytoarchitecture and transcriptional profiles of neocortical malformations in inbred mice. Cereb Cortex. 2008;18:2614–28. doi:10.1093/cercor/bhn019.
Mangaru Z, Salem E, Sherman M, Van Dine SE, Bhambri A, et al. Neuronal migration defect of the developing cerebellar vermis in substrains of C57BL/6 mice: cytoarchitecture and prevalence of molecular layer heterotopia. Dev Neurosci. 2013;35:28–39. http://www.ncbi.nlm.nih.gov/pubmed/23428637.
Reiss AL, Patel S, Kumar AJ, Freund L. Preliminary communication: neuroanatomical variations of the posterior fossa in men with the fragile X (Martin-Bell) syndrome. Am J Med Genet. 1988;31:407–14. doi:10.1002/ajmg.1320310220.
Webb SJ, Sparks BF, Friedman SD, Shaw DWW, Giedd J, et al. Cerebellar vermal volumes and behavioral correlates in children with autism spectrum disorder. Psychiatry Res - Neuroimaging. 2009;172:61–7. doi:10.1016/j.pscychresns.2008.06.001.
Acknowledgments
We thank Jeanne Quidore-Jermann and the staff of the New York Institute of Technology College of Osteopathic Medicine animal facility. This document has been subjected to review by the National Health and Environmental Effects Research Laboratory and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Conflict of Interest
We, the authors of this manuscript, certify that we have no financial or other conflict of interest related to the data contained in the following publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramos, R.L., Van Dine, S.E., Gilbert, M.E. et al. Neurodevelopmental Malformations of the Cerebellar Vermis in Genetically Engineered Rats. Cerebellum 14, 624–631 (2015). https://doi.org/10.1007/s12311-015-0657-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-015-0657-9