Abstract
Multiple myeloma (MM) is a neoplasm characterized by proliferation of clonal plasma cells (PCs) and a combination of clinical manifestations. Flow cytometry is an important method for diagnosing and monitoring of MM. Cytogenetic profiling of neoplastic PCs provides important prognostic information. Although stem cell transplantation (SCT) has significantly improved the overall survival of patients with MM, most SCT recipients relapse. We have studied the immunophenotypic and cytogenetic dissimilarities in the neoplastic PCs before SCT and after relapse in patients with initial complete remission, and investigated a possible influence of such dissimilarities on the patients’ survival. We retrospectively reviewed results of flow cytometric studies of bone marrow specimens from 46 patients with MM who underwent SCT, demonstrated a complete initial response, but subsequently relapsed. In nine of these patients, fluorescence in situ hybridization (FISH) studies were performed both pre-SCT and post-relapse. We have shown a significant flow cytometric and cytogenetic diversity of the neoplastic PCs in relapsed MM post-SCT. Such changes were detected in a considerable number of cases (47.8% and 44.4%, respectively). The most frequent cytogenetic changes indicate an emergence of possibly a more aggressive PC clone, known to be associated with worse prognosis and poorer outcome. Our study has demonstrated that the acquisition of immunophenotypic changes predicts worse overall survival.
Similar content being viewed by others
Introduction
Multiple myeloma (MM) is a malignant disease characterized by a proliferation of clonal plasma cells (PCs) and a combination of clinical manifestations, including the presence of monoclonal protein in serum and urine, anemia, hypercalcemia, abnormal renal function, and lytic bone lesions [1]. Flow cytometry remains an important method for diagnosing and monitoring of MM [2, 3]. The phenotypic profile of neoplastic plasma cells is variable; however, a commonly applied flow cytometric identification panel of markers includes CD45, CD138, CD38, CD56, and cytoplasmic immunoglobulin light chains [4]. In contrast to normal PCs (positive for CD19, CD45, bright CD38, and CD138 and negative for CD56), the neoplastic PCs are CD45 dim to negative, CD38 brightly positive, CD138 positive, CD19 negative, and frequently CD56 positive [5,6,7,8]. An additional panel of phenotypic markers that has certain prognostic and therapeutic significance is commonly performed. Among those are CD28, CD52, CD54, and CD117 [6, 9].
Cytogenetic profiling of the neoplastic PCs implicates important prognostic information [10,11,12]. Hyperdiploidy is associated with a more indolent form of the disease and demonstrates a tendency towards a more favorable outcome [13]. Equally important is the translocation status of the IGH gene, which can have numerous translocation partners with varying prognostic significance [11]. Cytogenetic studies are also helpful in determining disease progression. Although the specific steps of MM progression remain to be elucidated, certain genetic abnormalities have been shown to reflect the clonal evolution and disease progression. Deletion of 17p13 is considered to be an important factor for prognostication, because a loss of the tumor suppressor gene p53 has a negative effect on survival [14,15,16]. In addition, chromosome 1 abnormalities are also associated with shorter survival and are characterized by 1p deletion or 1q amplification [17,18,19].
The use of novel chemotherapeutic agents in combination with stem cell transplantation (SCT) has significantly increased the overall survival of patients with MM [20, 21]. However, the disease remains incurable and demonstrates a significant shortening of life expectancy. Unfortunately, most of SCT recipients, including those who demonstrate initial complete remission, relapse. A high rate of relapse has been attributed to clonal heterogeneity of the neoplasm as well as clonal evolution resulting in the persistence of a neoplastic clone. Current treatment considerations are restricted to a subset of patients with symptomatic relapse, advanced disease at diagnosis, or significant paraproteinemic increase [22]. Although neoplastic PCs frequently acquire additional mutations through the course of the disease, the changes in immunophenotypic and cytogenetic profiles in the context of relapsed MM in transplanted patients have not been reported, and the clinical significance of flow cytometric and fluorescence in situ hybridization (FISH) studies in such cases is lacking. Herein we attempt to evaluate immunophenotypic and cytogenetic dissimilarities in the neoplastic PCs before SCT and after relapse in patients who initially achieved a complete remission as well as investigate a possible influence of such dissimilarities on the patients’ survival.
Materials and methods
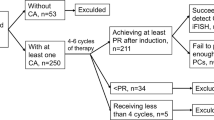
This study was approved by the Institutional Review Board at the University of Utah. We retrospectively reviewed results of flow cytometric and FISH studies of bone marrow specimens from consecutive 497 patients with an established diagnosis of MM who underwent bone marrow SCT between 2008 and 2015 at the University of Utah Hospital, Huntsman Cancer Institute. Of those, 46 individuals demonstrated a complete initial response, and subsequently met the clinical criteria for disease recurrence, and were included in this study. We performed a comparative analysis of flow cytometric and cytogenetic (FISH) profiles of pre-SCT and post-relapse bone marrow samples and evaluated the rates of overall survival.
Flow cytometric analysis
The bone marrow specimens of the study cohort were analyzed by five-color flow cytometry as a part of routine clinical assessment using a FC500 flow cytometer (Beckman Coulter, Miami, FL) by CXP software (Beckman Coulter). A panel of the following antibodies was used for flow cytometric evaluation of PCs: CD20, CD38, CD45, CD117, CD138 (Beckman Coulter, Immunotech), CD19 (Coulter Cyto-Stat), CD52, and CD54 (BioLegend, San Diego, CA), as well as immunoglobulin kappa and lambda light chains (Becton Dickinson, San Diego, CA). A combination of gating strategies was applied to every case to evaluate expression of the markers listed. The PC population was initially identified using CD45 versus light scatter plots in conjunction with cytoplasmic kappa and lambda immunoglobulin light chains. The neoplastic nature of the PC was detected by identifying of a population with bright expression of CD38, positive for CD138, immunoglobulin light chain restricted and frequent, but not ubiquitous aberrant expression of CD56. Neoplastic PCs were also studied for CD19, CD52, CD54, and CD117 expression. Expression of a marker was considered positive if it was present in > 20% of the neoplastic population. Upregulation of the antigen was defined as expression of an antigen not expressed in the initial sample or twofold or greater increase of neoplastic PCs expressing a particular antigen. Similarly, downregulation of the antigen was defined as loss of expression or twofold or greater decrease in PCs expressing of a particular antigen.
FISH analysis
The initial processing of the bone marrow specimen involved PC sorting using a column-free automated method, RoboSep (StemCell Technologies, Vancouver, BC, Canada), that enriched for CD138-positive cells. Following enrichment, the cellularity of the suspension was evaluated by phase-contrast microscopy. The FISH panel included the following probes: CKS1B, TP53 (CytoCell Ltd., Cambridge, UK), ASS1, CCND1/IGH XT, IGH break-apart, and PML (Abbott Molecular, Abbott Park, IL). When an IGH rearrangement and/or deletion were/was detected, additional FISH analysis with FGFR3/IGH and IGH/MAF probe sets (Abbott Molecular) was also performed. Two hundred interphase cells were evaluated for each probe set.
Statistical analysis
We created survival curves using the product limit method of Kaplan and Meier and compared them using logrank test and the Gehan-Wilcoxon test. The statistical analysis was performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, California). P values of less than 0.05 were considered to indicate statistical significance.
Results
Flow cytometric data for CD19, CD20, CD38, CD45, CD56, and CD138 were available for all 46 patients prior to SCT and after relapse. Only 19 patients were evaluated for CD52, CD54, and CD117 in both pre-SCT and post-relapse specimens. Of 46 patients, 22 (47.8%) demonstrated immunophenotypic dissimilarities of the neoplastic PCs in the relapsed disease compared to pre-SCT including six (13.0%) with a variable expression of at least two markers. Whereas CD45 and CD38 showed minimal variability, other markers indicated heterogeneity of their expression pattern (Fig. 1) with CD56 showing the most variability. Variation of CD56 expression was detected in 11 (23.9%) cases, including six (13.0%) cases with increased and five (10.9%) with decreased expression. Four of 19 cases (21.1%) demonstrated dissimilarities of CD117 expression with upregulation or downregulation in two (10.5%) patients each. CD20 and CD19 also demonstrated expression variations in five (10.9%) and four (8.7%) cases, respectively, with almost equal number of upregulated and downregulated events. CD52 and CD54 showed upregulation in one (5.2%) case each.
Immunophenotypic variability of neoplastic PCs in pre-CST (A) and relapsed (B) disease. (A) Neoplastic PCs in the pre-SCT specimen demonstrate kappa light chain restriction and CD56 positivity. (B) The same patient after post-SCT relapse: the neoplastic PCs are kappa light chain restricted but have lost CD56 expression
Pre-SCT and post-relapse FISH on CD138 sorted cells was available for nine patients. Of those, differences in the cytogenetic profile were detected in four (44.4%) cases. Two patients were found to have two co-existing cytogenetically distinct neoplastic subclones, one being similar to the pre-SCT, and the other with FISH differences. Two other patients accumulated additional cytogenetic aberrations with persistence of those seen in pre-SCT specimens. The most common patterns of post-relapse FISH dissimilarity were loss of previously detected hyperdiploidy, seen in three (33.3%) cases, and gain of 1q21 in three (33.3%) cases.
Comparing cases with immunophenotypic dissimilarities to those with cytogenetic differences, no distinct patterns of association were identified. Although, the majority of cases with FISH variabilities (3 of 4) also had immunophenotypic dissimilarities, different immunomarkers and different expression patterns were involved, in particular CD54, CD56, and CD117. Among five cases with an unchanged FISH profile, two showed immunophenotypic dissimilarities, including increased CD56 and decreased CD19 expression.
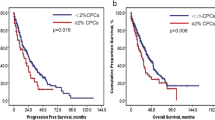
Multivariate modeling was performed using the parameters age, gender, flow cytometry markers, immunofixation, and serum protein electrophoresis: total protein, IgM, IgG, IgA, gamma, B2-microglobulin, alpha 2, alpha 1, albumin, serum free light chain, FISH MM panel, and cytogenetic results. In multivariate modeling with forward selection, the presence of differences in immunophenotypic profile was the only significant prognostic factor for overall survival (hazard ratio 0.13, 95% CI ratio 0.043–0.379, P < 0.0003) (Fig. 2). Due to a low number of observations, we were not able to demonstrate a statistically significant effect of particular patterns of immunophenotypic and/or cytogenetic changes on the overall survival, if such existed.
Discussion
Our study illustrates immunophenotypic and cytogenetic alterations in a considerable number of the relapsed MM cases (47.8% and 44.4%, respectively) compared to pre-SCT samples. The antigen expression frequency in pre-SCT MM in our study was overall similar to that reported in the literature [23] and showed the following pattern, in descending order: CD54 > CD56 > CD117 > CD52 > CD20 > CD19. The relapsed MM cases showed antigen frequencies without significant overall variation from the pre-SCT: CD54 > CD52 > CD117 > CD56 > CD20 > CD19 (Table 1). We found a slightly higher expression frequency of CD52 in both pre-SCT and post-relapsed cases than in some previous studies [23,24,25,26], which may reflect bias introduced by the selection of patients with relapsed and, therefore, more aggressive disease. Relapsed disease demonstrated changes in expression of the majority of antigens evaluated and involved CD56 > CD117 > CD20 > CD19 > CD54/CD52 > CD138, in descending order.
Immunophenotypic variability of neoplastic PCs is a well-documented event. Several reports pointed out to the existence of different subsets of myeloma PCs with unique patterns of chemoresistance [27]. Genetic heterogeneity has not only been recently demonstrated in newly diagnosed MM patients, but also at relapse, where the subclonal diversity of myeloma PCs often differs to that observed at diagnosis. Gupta and colleagues reported immunophenotypic changes following chemotherapy in as many as 78% cases [28]. Data on the subclonal phenotype distribution of myeloma PCs after response to first-line therapy is sparse to absent. However, to the best of our knowledge, this is the first study to report immunophenotypic and cytogenetic evolution patterns of the neoplastic PCs in multiple myeloma relapsed after SCT. We have shown a lower relative number of immunophenotypic changes (47.8%), most likely related to the differences in patient selection criteria or due to different modality of treatment with the major clone being extinguished by SCT in our patient population. Nonetheless, these findings in both studies support the concept of intraclonal heterogeneity of malignant cells and are consistent with a current and increasingly acknowledged hypothesis of MM being a composite disease [29], and that minor subclones of neoplastic PCs can provide a reservoir for relapse after the major clone is extinguished by chemotherapy [30] and SCT.
We have observed that relapsed MM often demonstrated acquisition of cytogenetic changes associated with an adverse prognosis (e.g., loss of hyperdiploidy and gain of 1q21). More importantly, high frequency of antigenic variation in the relapsed cases was associated with statistically significant worse outcome. This observation suggests a potential role in stratification of these patients to more aggressive therapy; however, further investigation in a larger cohort is required to establish clinical significance.
In conclusion, post-SCT relapsed MM demonstrates significant flow cytometric and cytogenetic diversity of the neoplastic PCs. The most frequent cytogenetic changes indicate an emergence of possibly a more aggressive PC clone, known to be associated with worse prognosis and poorer outcome. Our study showed that the acquisition of immunophenotypic changes has a negative effect on the overall survival. The clinical relevance of particular antigen expression patterns and the role of flow cytometric and repeated FISH testing in relapsed MM need to be elucidated in larger studies.
References
McKenna RW, Kyle RA, Kuehl WM, Grogan TM, Harris NL (2008) R.W. C: plasma cell neoplasms. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW (eds) Who classification of Tumours of Haematopoietic and lymphoid tissues. WHO Press, Lyon
Rawstron AC, Davies FE, DasGupta R, Ashcroft AJ, Patmore R, Drayson MT, Owen RG, Jack AS, Child JA, Morgan GJ (2002) Flow cytometric disease monitoring in multiple myeloma: the relationship between normal and neoplastic plasma cells predicts outcome after transplantation. Blood 100:3095–3100
Bakkus MH, Bouko Y, Samson D, Apperley JF, Thielemans K, Van Camp B, Benner A, Goldschmidt H, Moos M, Cremer FW (2004) Post-transplantation tumour load in bone marrow, as assessed by quantitative ASO-PCR, is a prognostic parameter in multiple myeloma. Br J Haematol 126:665–674
Raja KR, Kovarova L, Hajek R (2010) Review of phenotypic markers used in flow cytometric analysis of MGUS and MM, and applicability of flow cytometry in other plasma cell disorders. Br J Haematol 149:334–351
Paiva B, Almeida J, Perez-Andres M, Mateo G, Lopez A, Rasillo A, Vidriales MB, Lopez-Berges MC, Miguel JF, Orfao A (2010) Utility of flow cytometry immunophenotyping in multiple myeloma and other clonal plasma cell-related disorders. Cytometry B Clin Cytom 78:239–252
Leo R, Boeker M, Peest D, Hein R, Bartl R, Gessner JE, Selbach J, Wacker G, Deicher H (1992) Multiparameter analyses of normal and malignant human plasma cells: CD38++, CD56+, CD54+, cIg+ is the common phenotype of myeloma cells. Ann Hematol 64:132–139
Drach J, Gattringer C, Huber H (1991) Expression of the neural cell adhesion molecule (CD56) by human myeloma cells. Clin Exp Immunol 83:418–422
Hundemer M, Klein U, Hose D, Raab MS, Cremer FW, Jauch A, Benner A, Heiss C, Moos M, Ho AD, Goldschmidt H (2007) Lack of CD56 expression on myeloma cells is not a marker for poor prognosis in patients treated by high-dose chemotherapy and is associated with translocation t(11;14). Bone Marrow Transplant 40:1033–1037
Kumar S, Kimlinger T, Morice W (2010) Immunophenotyping in multiple myeloma and related plasma cell disorders. Best Pract Res Clin Haematol 23:433–451
Kastrinakis NG, Gorgoulis VG, Foukas PG, Dimopoulos MA, Kittas C (2000) Molecular aspects of multiple myeloma. Ann Oncol 11:1217–1228
Kuehl WM, Bergsagel PL (2005) Early genetic events provide the basis for a clinical classification of multiple myeloma. Hematology Am Soc Hematol Educ Program 2005:346–352
Kuehl WM, Bergsagel PL (2012) Molecular pathogenesis of multiple myeloma and its premalignant precursor. J Clin Invest 122:3456–3463
Smadja NV, Bastard C, Brigaudeau C, Leroux D, Fruchart C (2001) Hypodiploidy is a major prognostic factor in multiple myeloma. Blood 98:2229–2238
Fonseca R, Blood E, Rue M, Harrington D, Oken MM, Kyle RA, Dewald GW, Van Ness B, Van Wier SA, Henderson KJ, Bailey RJ, Greipp PR (2003) Clinical and biologic implications of recurrent genomic aberrations in myeloma. Blood 101:4569–4575
Avet-Loiseau H, Attal M, Moreau P, Charbonnel C, Garban F, Hulin C, Leyvraz S, Michallet M, Yakoub-Agha I, Garderet L, Marit G, Michaux L, Voillat L, Renaud M, Grosbois B, Guillerm G, Benboubker L, Monconduit M, Thieblemont C, Casassus P, Caillot D, Stoppa AM, Sotto JJ, Wetterwald M, Dumontet C, Fuzibet JG, Azais I, Dorvaux V, Zandecki M, Bataille R, Minvielle S, Harousseau JL, Facon T, Mathiot C (2007) Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood 109:3489–3495
Drach J, Ackermann J, Fritz E, Kromer E, Schuster R, Gisslinger H, DeSantis M, Zojer N, Fiegl M, Roka S, Schuster J, Heinz R, Ludwig H, Huber H (1998) Presence of a p53 gene deletion in patients with multiple myeloma predicts for short survival after conventional-dose chemotherapy. Blood 92:802–809
Walker BA, Leone PE, Chiecchio L, Dickens NJ, Jenner MW, Boyd KD, Johnson DC, Gonzalez D, Dagrada GP, Protheroe RK, Konn ZJ, Stockley DM, Gregory WM, Davies FE, Ross FM, Morgan GJ (2010) A compendium of myeloma-associated chromosomal copy number abnormalities and their prognostic value. Blood 116:e56–e65
Chang H, Qi X, Jiang A, Xu W, Young T, Reece D (2010) 1p21 deletions are strongly associated with 1q21 gains and are an independent adverse prognostic factor for the outcome of high-dose chemotherapy in patients with multiple myeloma. Bone Marrow Transplant 45:117–121
Shaughnessy J (2005) Amplification and overexpression of CKS1B at chromosome band 1q21 is associated with reduced levels of p27Kip1 and an aggressive clinical course in multiple myeloma. Hematology 10(Suppl 1):117–126
Corso A, Varettoni M (2007) The impact of new emerging drugs in the treatment of multiple myeloma: is there still a role for PBSC transplantation? Cur Stem Cell Res Ther 2:1–11
Srikanth M, Davies FE, Morgan GJ (2008) An update on drug combinations for treatment of myeloma. Expert Opin Investig Drugs 17:1–12
Blade J, Rosinol L, Fernandez de Larrea C (2015) How I treat relapsed myeloma. Blood 125:1532–1540
Cao W, Goolsby CL, Nelson BP, Singhal S, Mehta J, Peterson LC (2008) Instability of immunophenotype in plasma cell myeloma. Am J Clin Pathol 129:926–933
Lin P, Owens R, Tricot G, Wilson CS (2004) Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol 121:482–488
Kumar S, Kimlinger TK, Lust JA, Donovan K, Witzig TE (2003) Expression of CD52 on plasma cells in plasma cell proliferative disorders. Blood 102:1075–1077
Salama ME, Du S, Efimova O, Heikal NM, Wendlandt E, Toydemir RM, South S, Perkins SL, Hussong JW, Zhan F (2015) Neoplastic plasma cell aberrant antigen expression patterns and their association with genetic abnormalities. Leuk Lymphoma 56:426–433
Paíno T, Paiva B, Sayagués JM, Mota I, Carvalheiro T, Corchete LA, Aires-Mejía I, Pérez JJ, Sanchez ML, Barcena P, Ocio EM, San-Segundo L, Sarasquete ME, García-Sanz R, Vidriales MB, Oriol A, Hernández MT, Echeveste MA, Paiva A, Blade J, Lahuerta JJ, Orfao A, Mateos MV, Gutiérrez NC, San-Miguel JF (2015) Phenotypic identification of subclones in multiple myeloma with different chemoresistant, cytogenetic and clonogenic potential. Leukemia 29:1186–1194
Gupta R, Bhaskar A, Kumar L, Sharma A, Jain P (2009) Flow cytometric immunophenotyping and minimal residual disease analysis in multiple myeloma. Am J Clin Pathol 132:728–732
Greaves M, Maley CC (2012) Clonal evolution in cancer. Nature 481:306–313
Magrangeas F, Avet-Loiseau H, Gouraud W, Lode L, Decaux O, Godmer P, Garderet L, Voillat L, Facon T, Stoppa AM, Marit G, Hulin C, Casassus P, Tiab M, Voog E, Randriamalala E, Anderson KC, Moreau P, Munshi NC, Minvielle S (2013) Minor clone provides a reservoir for relapse in multiple myeloma. Leukemia 27:473–481
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Institutional Review Board at the University of Utah.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Toydemir, R.M., Rets, A.V., Hussong, J.W. et al. Immunophenotypic and cytogenetic evolution patterns of the neoplastic plasma cells in multiple myeloma relapsed after stem cell transplant. J Hematopathol 11, 75–80 (2018). https://doi.org/10.1007/s12308-018-0330-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12308-018-0330-6